Ammonia, chemical formula NH3, is a colorless gas frequently used in the production of fertilizer, as a cleaning chemical, and in the creation of nitrogenous compounds. You might be wondering, is ammonia a polar molecule or nonpolar molecule? The answer is that ammonia is a polar molecule, with its polarity being influenced by its asymmetrical shape and the presence of the nitrogen and hydrogen atoms within it. The nitrogen atoms within a molecule of ammonia have more electronegativity than the hydrogen atoms, which makes it a polar molecule.
“Life… is a relationship between molecules.” — Linus Pauling
While that is the short answer regarding the polarity of ammonia, to get a better understanding of how a molecule’s polarity is decided it is necessary to discuss how electronegativity, the shape of the molecule, and the types of atoms within the molecule all influence the polarity of a molecule.
Defining Polarity: What Does The Polarity Of A Molecule Refer To?
In everyday conversation, you are most likely to hear the word polarity being applied to the north and south poles on the Earth. These south and north poles are found at opposing points on the Earth’s surface, opposite one another. In a similar way, if you’ve examined a battery you’ve probably noticed that they have two ends: a positive end and a negative end. In the example of batteries, one end has a negative charge and the other has a positive charge. Atoms can have polarity too, and so can the bonds that exist between atoms, the bonds that create a molecule. A molecule is considered to be polar when the molecule’s constituent atoms are arranged in such a way that one end of the molecule has a net positive charge while a net negative charge is found on the other end of the molecule.
When an atom possessing a weak electronegativity level bonds with an atom that has a higher electronegativity level, the creation of a polar molecule occurs. A molecule with electrical poles results from the fusion of these two atoms, with one region having lower electronegativity and one region with higher electronegativity. Water is one of the most notable examples of a polar molecule on earth, and water’s polar nature means that it can bond with many other molecules. This attribute enables it to serve as the basis for life on the planet.
Non-polar molecules don’t have electrical poles, in contrast to polar molecules. Nonpolar molecules also have electrons that have a more even distribution, electrons distributed more equally. Because of the even distribution of electrons, molecules which are nonpolar don’t have a notable charge on either end of the molecule. Nonpolar molecules include most hydrocarbons.
In essence, a nonpolar molecule is where the molecule lacks a significant net positive charge on one end and a negative charge on the other end. The charges of the atoms cancel out in the case of nonpolar molecules. By contrast, polar molecules have dipoles that have a net positive charge and a net negative charge, charges that don’t cancel each other out.
Examples Of Polar Molecules
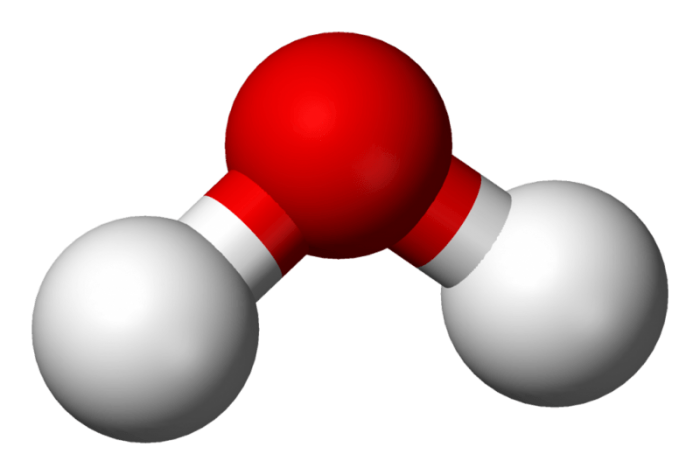
Photo: By Benjah-bmm27 – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=1997535
The bonds that are found between the hydrogen and oxygen atoms in a molecule of water are distributed in a fashion such that there is equal space on both sides of the hydrogen-oxygen bonds. Because of the fact that the bonds in water are equally spaced, one half of the molecule has a net positive charge while half the molecule has a net negative charge.
“Each one of us is a set of shifting molecules, spinning in ecstasy.” — Jim Jarmusch
Another example of a polar molecule is ethanol, which is polar due to the fact that the oxygen atoms found within ethanol have a higher electronegativity level than the atoms surrounding it. Ethanol has oxygen atoms in it, and these oxygen atoms have a greater electronegativity potential, meaning that they attract more electrons than the hydrogen atoms in the molecule. While ethanol doesn’t have a very high negative charge, the abundance of electrons means that the -OH bonds do possess a negative charge overall. Beyond ethanol and water, other polar molecules are hydrogen sulfide and sulfur dioxide.
Looking for the presence of polar bonds in a molecule is not a surefire way to determine if the molecule is polar or not. This is because it is possible for a molecule to be nonpolar yet still have polar bonds. One example of this phenomenon is carbon dioxide. Although there are polar bonds found within a carbon dioxide molecule, the molecule overall is nonpolar because the dipole moments of the molecule neutralize one another.
Examples Of Nonpolar Molecules
Nonpolar molecules are molecules like oxygen, nitrogen, and ozone. These specific nonpolar molecules are comprised out of a single element. Molecules comprised of only a single type of atom are referred to as homonuclear molecules. Molecules that aren’t homonuclear but that are nonpolar include methane, carbon tetrachloride, and carbon dioxide. Gasoline, acetic acid, and toluene are also nonpolar substances.
Photo: By Benjah-bmm27 – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=2062504
Carbon compounds are nonpolar in general, with carbon monoxide being an exception to this rule. The structure of carbon monoxide makes it a polar molecule. The electronegativity difference between the carbon molecules and oxygen in a molecule of carbon monoxide is enough that the molecule is polar. Carbon monoxide is linearly structured and although most linear molecules are nonpolar, CO is an exception. Nonpolar molecules also include alkynes and noble gases. Alkynes don’t dissolve in water, while the noble gases or inert gases are classified nonpolar due to the fact that the gases are made completely out of atoms of the same element. The elements making up the noble gases include krypton, neon, argon, and helium.
Predicting The Polarity Of A Molecule
Determining the electronegativity values of the atoms within a molecule/calculating the electronegativity of atoms is a key aspect of determining whether a molecule is polar or nonpolar. If there are substantial differences in the electronegativity values between atoms, the electrons are likely to be unequally shared between the atoms. The electrons will be closer to one atom than to another if there are large differences in electronegativity, and that region of the molecule will be polar in nature. However, the electronegativity of all bonds must be taken into account to determine if a molecule is nonpolar or polar.
“In inorganic chemistry the radicals are simple; in organic chemistry they are compounds – that is the sole difference.” — Jean-Baptiste Dumas
A molecule’s geometry is one of the deciding factors in a molecule’s polarity. The molecule will be polar if one end has a negative charge while the other end has a positive charge. However, if the charges in a molecule are orbiting a central atom and are evenly distributed then the molecule is likely nonpolar. These attributes must be considered together when attempting to predict whether or not a molecule is nonpolar or polar. Because not all molecules have dipole moments predicting the polarity of a molecule can be challenging. For example, molecules that can be mirrored/flipped across a geometric plane don’t have dipole moments, because dipole moments are just a single point.
Facts About Ammonia
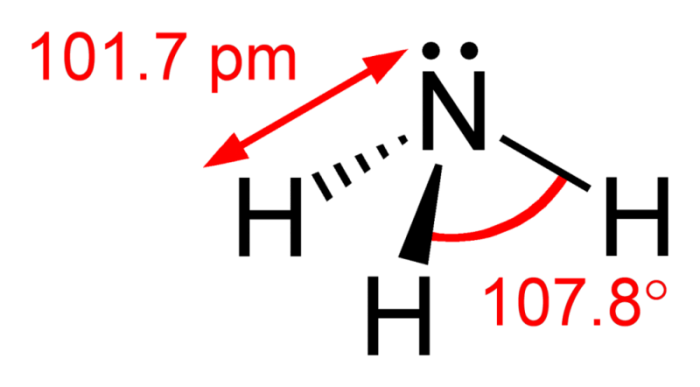
Photo: By Ben Mills – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=6628922
Ammonia has a powerful, pungent smell. In concentrated forms, ammonia is hazardous and caustic. Ammonia is a common ingredient in the creation of many different pharmaceutical products. Household ammonia, a specific formula known as ammonium hydroxide, is ammonia dissolved into water. Regular ammonia boils at −33.34 °C (−28.012 °F), and this extremely low boiling point means that in order for liquid ammonia to be kept it must be stored at very low temperatures or under pressure. Ammonia is easy to liquefy because of the fact that ammonia molecules have a strong hydrogen bond. Liquid ammonia is useful as a solvent because it possesses powerful ionizing abilities. Water is miscible with ammonia, and when ammonia and water are mixed together the ammonia can be removed from the water through boiling.
“Stability can only be obtained by inactive matter.” — Marie Curie
Ammonia occurs in nature but only in trace amounts. Natural ammonia is produced by vegetable matter and nitrogenous animals matter. One of the primary uses for ammonia is in the creation of fertilizer. around 89% of all ammonia is applied to fertilizers, creating salts or solutions. The fertilizers created with ammonia help increase the yield of crops. Ammonia is also frequently used in household cleaners, frequently for porcelain and glass surfaces. Household ammonia is diluted in water, and typically has a concentration of 5 to 10% of ammonia per container of cleaner.
Ammonia has a chemical formula of NH3, meaning there are three hydrogen atoms for every atom of nitrogen. Ammonia molecules are laid out as a trigonal pyramid, with the nitrogen atom at the top of the molecule and the hydrogen atoms linked at right angles. Taking both the strength of the electronegativity between bonds and the structure of the molecule into leads to NH3 being identified as polar. This is because NH3 has four electron groups, and one of those groups does not share its electrons. The molecule has an unshared and well as shared electron pairs, so it is polar.









