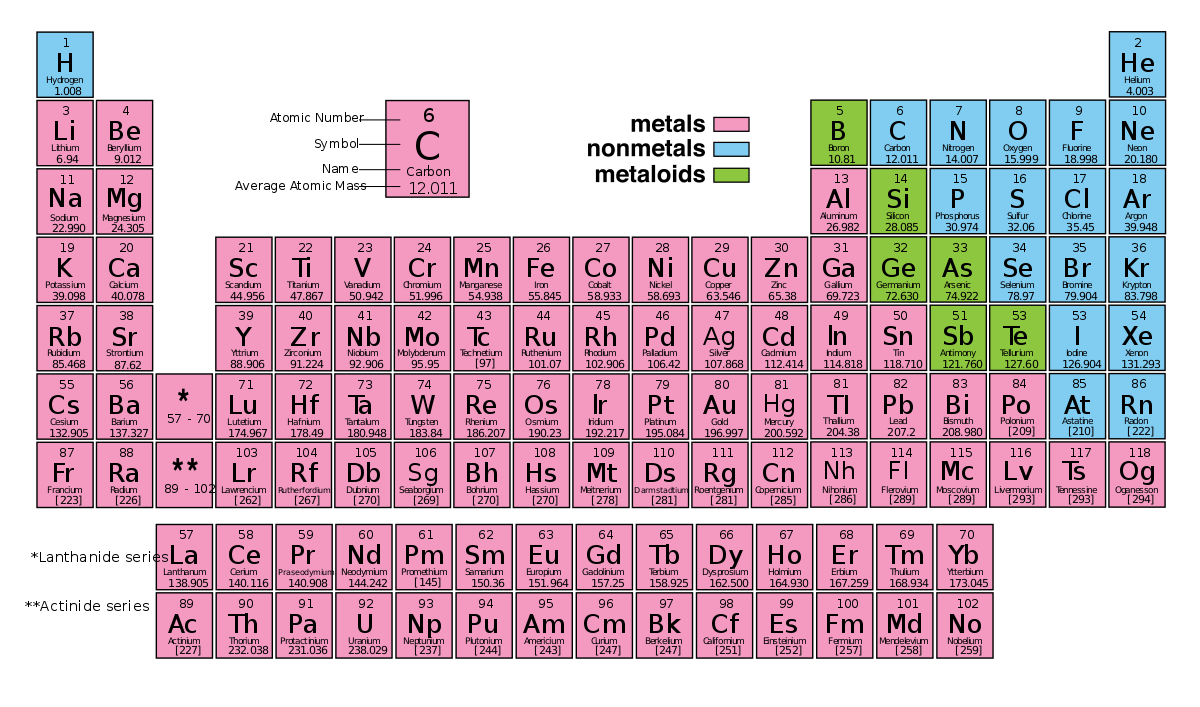
The periodic table of the elements is a representation of all of the chemical elements that have been discovered. The elements on the periodic table run from top to bottom and left to right in order of increasing atomic number, and in general the order of the elements is correlated with their atomic mass. In this article, you will find a labeled periodic table of the elements, as well as information about the trends relating to the periodic table, or the patterns you can use to better understand the table.
Interpreting The Periodic Table
Rows on the periodic table are referred to as periods, while the columns on the periodic table are referred to as groups. An element’s period number represents the highest energy level that an electron in that element possesses. Elements found within the same column have electron distributions that are identical, and because of this, they have very similar chemical properties and reactions.
Trends Of The Periodic Table
Ionic Radius Trend
The ionic radius trend describes how as one follows the periodic table from top to bottom, the ionic radius of the elements within the table tends to increase. Ionic radius tends to decrease as one reads across the table from left to right.

Photo: Photo: (https://pixabay.com/illustrations/periodic-system-chemistry-science-1059755/) by Geralt via Pixabay, Pixabay License (https://pixabay.com/service/license/)
What is the ionic radius exactly? As a quick definition, an atom’s ionic radius is a measurement of the ions taken as the atom is positioned inside of the crystal lattice. To be more concrete, the ionic radius of an atom is defined as half the distance between ions that are just barely contacting each other. The precise electron shell boundary for two different atoms is quite difficult to precisely measure, and so an atom’s ions are usually described as if they were solid. The ionic radius differs from the atomic radius in the fact that the atomic radius is the radius possessed by a neutral atom, and the ionic radius can be a little larger or little smaller than this.
The size of the ionic radius influences the type of particle charge the ion possesses. Positively charged ions, or cations, are typically smaller than an element’s neutral atom. This is due to the fact that an electron has been removed from the atom’s shell and the electrons surrounding it experience a greater attraction to the nucleus as a result. Meanwhile, a larger ionic radius typically indicates a negatively charged ion, also called an anion.
Ionization Energy Trend
The Ionization Energy Trend typically follows a noticeable pattern or trend across the periodic table. Ionization energy tends to decrease as one moves from top to bottom down the periodic table, and increase as you move from left to right across the table.
The term ionization energy refers to the total quantity or amount of energy that has to be absorbed by an ion to make it discharge an electron. In the case of an atom that is initially neutral, it typically requires less energy to discharge the first electron than it does the second electron, and it takes more energy to discharge the third electron than it does to discharge the second electronic, and so on.
The reason it requires more energy to remove every subsequent electron from an ion is that as electrons are removed, the ion becomes more positive. The greater the positivity of the ion, the more electrons are attracted to the ion, and so separating electrons from the ion comes increasingly difficult. The greater the distance between the electrons and the nucleus of the atom the simpler it is for an electron to be removed from the atom. As such, ionization energy is considered related to atomic radius.

Photo: Photo: (https://pixabay.com/illustrations/periodic-system-chemistry-science-1059755/) by Geralt via Pixabay, Pixabay License (https://pixabay.com/service/license/)
Electron Affinity Trend
Electron affinity refers to how much energy is spent or released when electrons are added onto a neutral atom, or to put that another way it refers to how much energy changes whenever a neutral atom has an electron added to it.
The Electron Affinity Trend refers to how the periodic table has a trend that sees electron affinity increasing as the table moves left to right. The complementary part of the electron affinity trend is that electron affinity generally decreases as the periodic table moves from top to bottom. The ions of atoms can possess a net negative charge or a net positive charge, and as previously discussed ions with a net negative charge are called anions while positively charged atoms are referred to as cations.
Here’s the distinction between electron affinities and ionization energies: ionization energies cover the formation of positive ions while electron affinities cover negative ions. The use of electron affinities is almost always restricted to the elements located in groups 16 and 17.
An atom’s electron affinity depends on when the electron is appended to the atom. When the first electron is added to a neutral atom, it will always possess negative energy and this is the first electron affinity. The reason this is so is that energy is released as the neural atom has an electron added, making the ion negative. More and more energy is necessary to add additional negative ions. Because the energy necessary to add an ion overpowers the energy released by the electron attachment process, the second electron affinity will be positive.
The Electronegativity Trend
The Electronegativity Trend refers to how electronegativity levels change as you follow the periodic table left to right or top to bottom. In general, electronegativity levels tend to increase as you follow the periodic table from top to bottom and it also increases as you move across the periodic table from left to right.
Electronegativity refers to the how much influence or “attractiveness” an atom has to the electrons which are found in a chemical bond or to put that another way it describes an atom’s ability to attract electrons when that atom is part of a given compound. Polar covalent bonds are created when the electrons that comprise a chemical bond have a weaker attraction to one atom than to another atom. In contrast, a covalent bond is created when two atoms have the same electronegativity values, thus sharing the electrons exactly.
If the electronegativity difference between two atoms is extremely great, the two atoms won’t share the electrons between them at all. The atom that has the greater electronegativity basically steals the electron from the other atom, and as a result, an ionic bond is created.
The Pauling Scale is a scale that tracks how strong the bond energies, the electronegativity values, for different atoms are. Atoms have electronegativity values between 0.7 to 3.98 and hydrogen, which has a value of 2.20, is used as the basis for the scale.
The Atomic Radius Trend
The term “atomic radius” describes the size of an atom. Atomic radius can be difficult to precisely measure, do in large part to the fact that there are competing ways to define atomic radius. The different ways to measure atomic radius include the Van der Waals radius, the covalent radius, the metallic radius, and the ionic radius.

Photo: A portrayal of the structure of a helium atom. Yzmo via Wikimedia, CC-BY-SA 3.0
The Van der Waals radius describes the distance in a defined element that exists between the closest approach of two non-bonded atoms. The metallic radius is taken when two metal ions are in a metallic lattice and it is half the distance between these two metal ions. The covalent radius describes the size of an atom which comprises a portion of a given covalent bond. Finally, the ionic radius is taken when a crystal lattice holds the ion and it is half the distance between the two nuclei of two different ions that are barely touching.
The Atomic Radius Trend refers to the fact that as you follow the periodic table down a group of elements, atomic radius tends to increase, which reflects the fact that as you move down a column another electron shell is added to the atom for every new row in the periodic table. Due to the fact that there is an increased number of electrons per atom as the atomic number goes up, the atomic radius tends to drop as you move left to right across the periodic table.
Metals, Metalloids and Nonmetals
One way that the elements of the periodic table are grouped together is through various properties that classify them one of three different groups: metals, metalloids/semimetals, and nonmetals.
Most of the elements found on the periodic table are metals, and metals are distinguished by properties like:
- Being shiny, having high luster
- Typically solid at room temperature
- Good conductors of electricity and heat
- Malleable
- Dense
- Loose electrons easily
Semimetals, or metalloids, have properties which are in between metals and nonmetals. These properties are:
- Can be either dull or shiny
- Often effective semiconductors
- Can lose or gain electrons in chemical reactions
- Have several different forms
Nonmetals are elements that have properties which are quite distinct from metals. The noble gasses, like oxygen, are nonmetals. Nonmetals have characteristics like the following:
- Often brittle
- Compared to metals they have low melting points
- Do not conduct electricity or heat very well
- Compared to metals they are much less dense
- Often gain electrons when involved in chemical reactions
- Appear dull, and not shiny









