Graphene has transformed material research worldwide. Graphene is considered an innovative material that could impact a wide variety of fields. The electronic, chemical, and structural aspects of graphene are responsible for its widespread application possibilities. Graphene, an attention-grabbing allotrope of carbon, belongs to the two-dimensional (2D) class of materials.
Recognition of the application scope of graphene has indeed popularized 2D materials and motivated researchers to find new layered structures. Graphene has a peculiar honeycomb framework formed with sp2 hybridized carbon atoms [1]. Its electrical conductivity and thermal resistance make graphene an excellent choice for electronic devices, storage applications, etc. The possibility for π conjugation in graphene makes the loading of aromatic drugs possible for drug delivery. Notably, graphene has been explored in photothermal therapy [2].
Along with graphene, other graphene-related materials like graphene oxide (GO) and reduced graphene oxide (rGO) is also gaining interest. Graphene oxide is the oxidized form of graphene. Reduction of GO by different reducing agents yields rGO with properties comparable to pristine graphene. rGO possesses defects when compared to graphene obtained from graphite. But rGO makes it possible to obtain graphene in bulk at low cost. Hence, rGO has been extensively explored for various applications. Different reducing agents, such as hydrazine hydrate, ascorbic acid, NaBH4, etc., are used for GO reduction (3). Availing toxic reducing agents like hydrazine hydrate is disadvantageous since it is explosive and difficult to use.
On the other hand, ascorbic acid provides a mild reducing environment which can effectively reduce GO to rGO. rGO produced from reducing GO with ascorbic acid exhibited properties as good as that of pristine graphene. The low-cost of synthesis and better control over reaction conditions has made ascorbic acid an ideal reducing agent for rGO synthesis (4).
rGO is synthesized with the interest of obtaining the same electronic properties of pristine graphene. At the same time, reducing GO to rGO causes increased hydrophobicity due to reduced oxygen functional groups eventually leading to aggregation. The lower water stability affects its applicability in biomedical applications. Functionalizing rGO surface is one way to obtain stable water suspension of rGO with the added advantage of lowering toxicity and improving compatibility with living cells (5). Surfactants and polymers, like polyethylene glycol (PEG), dextran, polyvinyl alcohol, pluronic, etc., are extensively used for functionalizations. Surface functionalization with a pluronic, structural triblock copolymer consisting of poly(ethylene oxide)-block-poly(propylene oxide)-block-poly- (ethylene oxide) yields an appreciably stable water dispersion of rGO (6).
Ascorbic acid reduction of GO to rGO and subsequent functionalization using pluronics produced a pluronics-stabilized rGO with a yield above 80%. The characterization of the synthesized pluronics stabilized rGO using different techniques like FT-IR, XRD, Raman spectroscopy, XPS, TGA, TEM, and XPS confirmed the successful reduction of GO to rGO as well as effective conjugation of pluronics to the rGO surface. Apart from the physicochemical characterization, the biological characterization of nanoparticles is also vital.
Endotoxin detection is a major biological characterization technique for nanoparticles. The nanoparticles need to be endotoxin free before biological assays since the interference of endotoxin may give false results. The endotoxin assessment of rGO and pluronics-stabilized rGO revealed that the endotoxin limit for both the nanoparticles was less than the USP recommended endotoxin level for drugs and medical devices (0.5 EU/ml). Hence, both rGO and pluronics-stabilized rGO are advisable for biomedical applications after a more thorough investigation.
The protein corona formation determines the fate of the nanoparticle when administered inside the body and is regarded as an important biological characterization technique (7). Proteins and biomolecules get adsorbed onto the surface of nanoparticles according to the Vroman effect, forming a hard and soft corona. The protein corona formed determines the biological response of the nanoparticle’s circulation, metabolism, and excretion. The SDS analysis of rGO and pluronics-stabilized nanoparticles revealed that rGO had a greater protein binding ability. No specific bands were observed in pluronics-stabilized rGO, implying low protein adsorption. Low protein adsorption may be attributed to the presence of PEG moiety in pluronics. PEG causes lower protein binding and makes penetration through the reticuloendothelial system possible (8).
The blood-brain barrier (BBB) is an important confinement that protects the nervous system. At the same time, the BBB hinders drug delivery at the neuronal site. The competence of nanoparticles to cross the BBB makes them reliable for diverse neuronal applications (Figure 1).
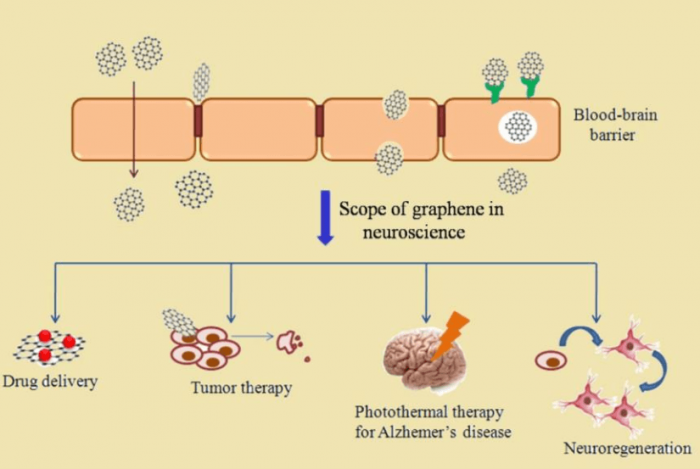
Figure 1: Various applications of graphene in neuroscience. Figure courtesy Mohanan PV.
Graphene, as per literature reflections, is optimal for drug delivery, deep brain stimulation, targeted therapy, etc (9). Attractively, graphene has been widely explored for the possibility for the photothermal treatment of Alzheimer’s disease (10). Apart from the ability to cross the BBB, graphene can also induce proliferation and differentiation of stem cells to neurons, a property fundamental for neuron regeneration. Validating the biocompatibility of graphene is necessary before its use in biomedical applications. rGO, as well as pluronics-stabilized rGO, need to be marked safe against any particle-induced neurophysiological changes. Hence PC-12 cells, a rat pheochromocytoma cell line, were chosen to analyze the effect of both rGO and pluronics-stabilized rGO (Figure 2).
PC-12 has the ability to differentiate neurons in response to nerve growth factor (NGF). MTS assay analysis showed dose-dependent toxicity when PC-12 cells were treated with rGO and pluronics-stabilized rGO. According to the lactate dehydrogenase (LDH) assay results, rGO induced membrane disruption as evident from the increased LDH release. On the other hand, pluronics-stabilized rGO did not induce any notable elevation in LDH release. So, it can be inferred that pluronics stabilization diminished the membrane destructing tendency of rGO.
The cytoskeletal integrity of rGO and pluronics-stabilized rGO treated PC-12 cells were analyzed by β-III tubulin staining. rGO induced cytoskeletal disruption at higher concentrations. No evident cytoskeletal disruption was detected in cells treated with pluronics-stabilized rGO. Oxidative stress is the major contributor to nanoparticle-induced toxicity.
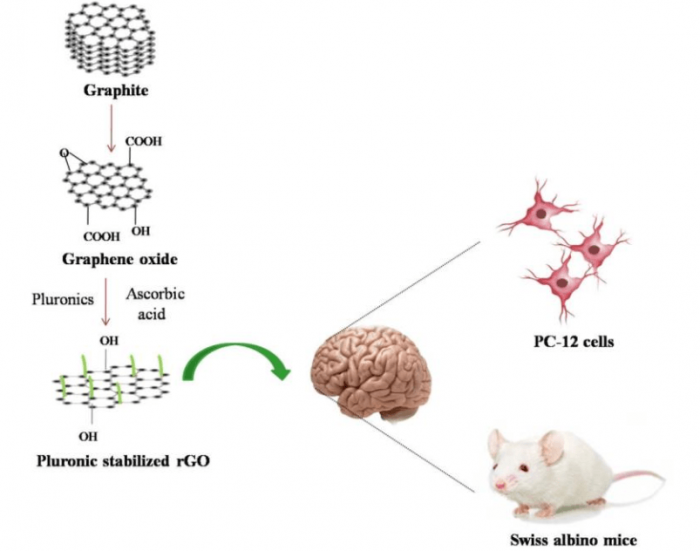
Figure 2: Synthesis of pluronic-stabilized rGO by reduction of GO using ascorbic acid and functionalization using pluronics. Evaluation of toxicity of pluronic stabilized rGO in an in vitro and in vivo system. Figure courtesy Mohanan PV.
For that reason, the ROS generation induced by nanoparticles needs to be monitored to have an idea about its associated toxicity. rGO did not endorse ROS generation in PC-12 cells. The hydrophobicity of rGO restricts its interaction with cells. That could be the reason for lower ROS detection in rGO treated cells. The radical scavenging behavior of graphene also helps in reducing ROS generation (11). PC-12 cells treated with pluronic-stabilized rGO showed a higher amount of ROS in comparison to untreated cells. The colloidal stability of pluronic-stabilized rGO facilitates better cellular interactions that responsively cause an increase in ROS with increasing dose concentrations (Figure 3).
Apart from the in vitro model, the effect of rGO and pluronics-stabilized rGO at the in vivo level was also analyzed. Swiss Albino mice were administrated with 10mg/ml concentrations of rGO and pluronics-stabilized rGO by both intravenous (i.v) and intraperitoneal (i.p) routes. rGO and pluronics-stabilized rGO didn’t induce any detectable toxicity up to 72h after administration. No behavior changes or weight loss was observed in treated mice.
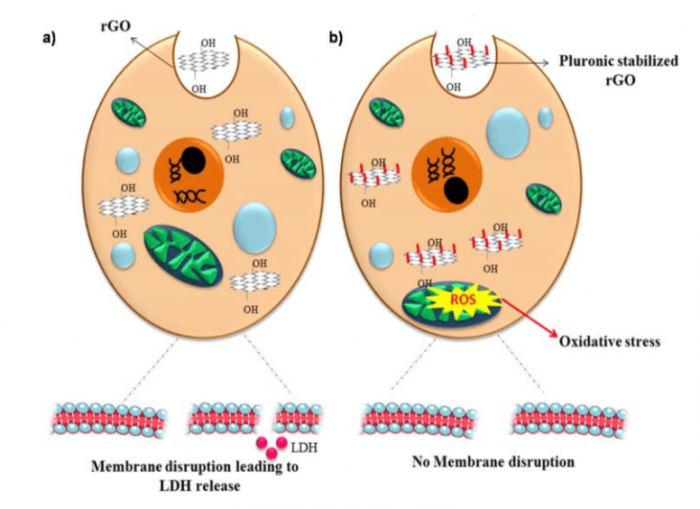
Figure 3: Cellular response when PC-12 cells were treated with a) rGO and b) pluronic-stabilized rGO. Figure courtesy Mohanan PV.
The results put forward an eco-friendly, reliable, and low-cost route for obtaining stable rGO dispersion by means of pluronics stabilization. Though the nanoparticle showed dose-dependent toxicity at the in vitro level, no substantial toxicity was detectable at the in vivo level. The proposed material needs to be further examined for repeated exposure to get a clearer picture of its scope in biomedical applications.









