
Ionization energy trend refers to the energy needed to displace an electron from a given atom, or the amount of energy required to remove an electron from an ion, or gaseous atom. Ionization energy is measured by the energy unit kilojoules per mole, or kj/mol.
The trend in ionization energy refers to how ionization energy follows a notable trend across the periodic table of the elements. Ionization energy typically increases as you move left or right across a row or element period, and it typically decreases as you move top to bottom down a column or element group.
Defining Ionization Energy
As mentioned, the ionization energy is the amount or quantity of energy that must be absorbed by an ion or isolated gaseous atom to discharge an electron. The discharge of an electron from a gaseous atom creates a cation or positively charged ion.
If an atom is initially neutral then discharging the first electron typically requires less overall energy than discharging the second electron. This continues to hold true for subsequent electrons. Displacing the second electron requires less energy than the third electron, and displacing the third electron requires less energy than discharging the fourth electron, and so on. This means that more energy is required to displace every subsequent electron.
“The removal of an electron from the surface of an atom – that is, the ionization of the atom – means a fundamental structure change in its surface layer.” — Johannes Stark
The reason this is true is that once the first electron is removed from the ion, the net charge of the atom is positive. The positive nature of the atoms means that the electron’s negative forces are attracted to the new ion’s positive charge, and this effect continues to multiply as more electrons are lost from the ion, so it becomes ever more difficult to separate the electrons from the ion.
The greater the distance between the nucleus and an electron, the easier it is for that electron to be displaced. For this reason, ionization energy is often considered a function of the atomic radius. The greater the atomic radius, the less energy is necessary to pry the electron from the outermost orbital or valence shell. As an example, it would be more difficult to remove an electron from an element like Chlorine, where the electrons are closer to the nucleus than removing an electron from an element like Calcium where the electrons are farther away from the nucleus.
The electron orbitals of atoms are separated into different shells. These shells have noticeable impacts on the amount of energy required to remove an electron from the atom. As an example, Aluminum is the first element in its row that has electrons in its 3rd shell, and it only needs to shed one electron to create a stable 3rd shell. This means that the ionization energy level of aluminum is fairly low when you compare it to other elements in that row. Remember that when dealing with subsequent ionization energies, there’s a large increase in the amount of ionization energy needed to expel an electron. In this case, trying to remove another electron means removing it from a pretty stable 3rd electron shell.
The Ionization Energy Trend
Ionization energies are dependent on the atomic radius of the atom in question. Ionization energies have an inverse relationship with the atomic radius. This means that since the atomic radius trend describes how moving from right to left on the periodic table increases the atomic radius, ionization energy increases as you move left or right. Ionization energy also decreases as you follow the table from top to bottom. There are a few notable exceptions to the ionization trend, such as the alkaline earth metals found in Group 2 and the Nitrogen group elements found within Group 15.

Note that groups run right to left while periods run top to bottom. Photo: ExplorersInternational via Pixabay
Group 2 and Group 15 of the periodic table have completely filled and half-filled shells respectively. The Group 2 elements usually have ionization energy which is greater than the elements found in group 13, while elements in group 15 have higher ionization energy than elements in group 16.
“Measurement of the specific ionization of both the positive and negative particles, by counting the number of droplets per unit length along the tracks, showed the great majority of both the positive and negative particles to possess an electric charge.” — Carl David Anderson
The group with the smallest overall ionization energies are the alkali metals, just compare them to the ionization energies for halogens. While the atomic radius plays an important role in determining the ionization energy of an atom, the number of electrons found between the nucleus and the electrons in the valence shell also has an impact on the ionization energy level.
The term shielding describes the phenomenon where the positive charge of an atom’s nucleus has less impact on the outer electrons since the negative charges found within the inner electrons reduce the effect of the positive charge. More electrons found in between the nucleus and the outer shell means more electrons to shield the outer shell from the effects of the nucleus, which translates to less energy needed to remove an electron from that atom. In other words, the greater the shielding effect is, the lower the ionization energy of that atom. This is why the ionization energy decreases as you move down a group. In terms of examples, Fluorine has the highest overall ionization energy while Cesium possesses the lowest overall ionization energy.
The Ionization Energy Trend and the Electron Affinity Trend
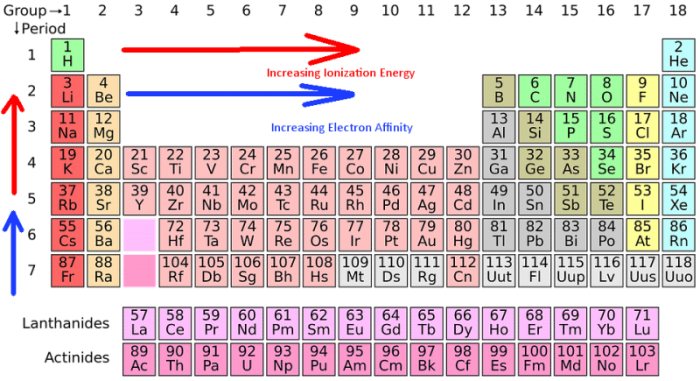
Photo: Geralt via PIxabay, CC0
Electron affinity and ionization energy both have similar trends you can find on the periodic table. The electron affinity of an atom increases along the periods on the table just as ionization energy also increases. Similarly, electron affinity decreases from top to bottom down the table just as ionization energy does. The cause of the decrease in both electron affinity and ionization energy is the same as well, the shielding effect. When you compare them with elements found in the first and second groups of the table, halogens are capable of easily capturing electrons. The tendency to attract and capture electrons when the element is in a gaseous state is referred to as electronegativity, and it has its own trend on the periodic table. Electronegativity also goes into determining the chemical differences that exist between metallic and non-metallic elements.
“I realized it was like a dating agency: the ions are the lost souls looking for mates; the electrolyte is the agency that can help them find each other.” — Victoria Finlay
To Sum Up:
Ionization energy refers to the minimum amount of energy needed to separate an electron from an ion or atom in its gas phase. Ionization energy is measured in either kilojoules per mole (kJ/M) or electron volts (eV). Ionization energy increases as you go left to right across a period on the table, and decreases as you move top to bottom down the table.









