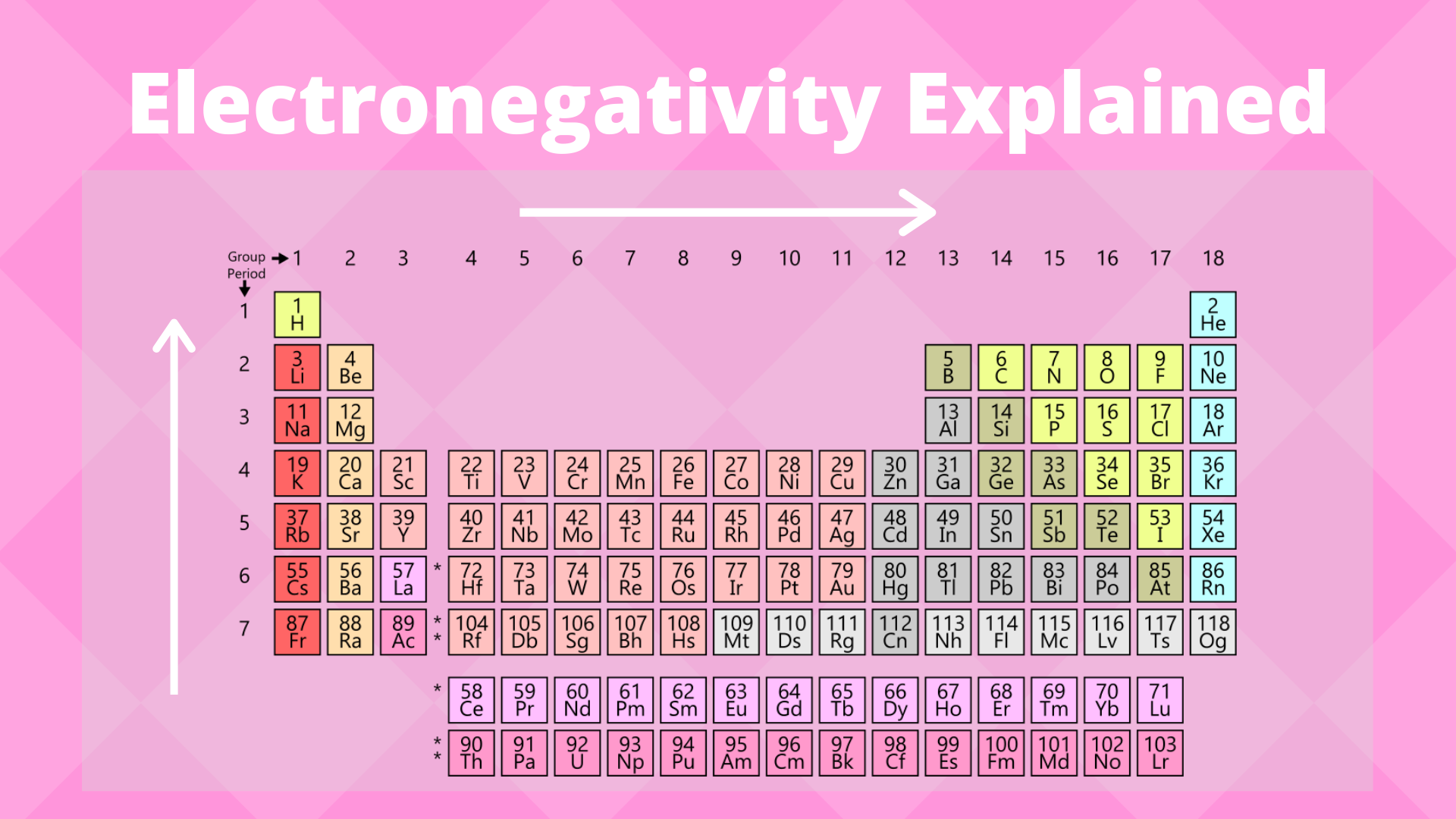
The electronegativity trend refers to a trend that can be seen across the periodic table. This trend is seen as you move across the periodic table from left to right: the electronegativity increases while it decreases as you move down a group of elements.
While this is the basic definition of the electronegativity trend, to truly understand it, it would be helpful to put it in perspective and look at some specific examples of the trend.
What Is An Electronegativity Trend?
Before we can begin looking at examples of the electronegativity trend, let’s define our terms. What is electronegativity exactly? Electronegativity refers to an atom’s ability to attract the electrons present in a chemical bond, or an atom’s ability to attract electrons when that atom is part of a specific compound. In most cases, the electrons found within a chemical bond have a greater attraction to one atom than to the other atom, which creates a polar covalent bond. Though sometimes two atoms will have the exact same electronegativity values and have a covalent bond, meaning that they equally share the electrons.
If two atoms have electronegativity values that are extremely different, they won’t share electrons between them at all. The atom with the greater value will basically take the electron bond from the other atom and possess it, creating an ionic bond.
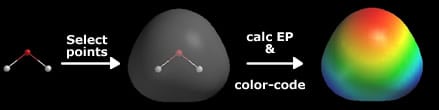
A visualization of a molecule’s electrostatic potential. Photo: FrozenMan at English Wikipedia, CC0, Public Domain
There is an electronegativity scale that reflects how strong the bond energies for atoms are. The scale is called the Pauling Scale, named after Linus Pauling who created the scale in 1932. The Pauling scale assigns atoms electronegativity values between 0.7 and 3.98. Hydrogen is used as the base for the scale, and it possesses an electronegativity value of 2.20. Though the Pauling scale is the most commonly used electronegativity scale, other scales like the Allen scale of the Mulliken scale do exist.
Remember that electronegativity emerges as a property of atoms within molecules and that it isn’t a property that’s inherent to atoms themselves. For this reason, the electronegativity value can change depending on the environment the atom is in. Most of the time though, atoms have similar behavior even in different environments. Factors which can influence the electronegativity value include the number of electron locations in an atom as well as the nuclear charge.
To put that another way, electronegativity isn’t measured in a standard like units of energy, it’s measured on a relative scale. This makes it different from electron affinity because electron affinity refers to the actual energy released when atoms end up gaining an electron.
The Electronegativity Trend
As a practical example of electronegativity in action, consider the fact that an atom of chlorine has a higher electronegativity value than an atom of hydrogen. The electronegativity of chlorine is 3.16 while, as previously mentioned, the electronegativity value of hydrogen is 2.20. This means that the electrons in the bond will be closer to the chlorine atom than the hydrogen atom in a molecule of HCl.
As mentioned, the electronegativity trend refers to the way electronegativity values trend across the periodic table of the elements. When moving from left to right across the periodic table, electronegativity increases, with the exception being the noble gases. In general, electronegativity decreases as you move down a group in the periodic table, this correlates neatly with the increase in distance between the atom’s nucleus and the electron valence.

Groups run left to right on the periodic table, while periods run top to bottom. Photo: Geralt via Pixabay, CC0
There are also other examples of exceptions to the electronegativity trend, these include lanthanides and actinides. This is true because the noble gases usually have a valence shell that is already full and thus can’t usually attract electrons. lanthanides and actinides are just more complicated chemicals that don’t really follow any trends.
“For me too, the periodic table was a passion. … As a boy, I stood in front of the display for hours, thinking how wonderful it was that each of those metal foils and jars of gas had its own distinct personality.” — Freeman Dyson
The element with the highest electronegativity value is fluorine, which has a rating of 3.98. The element with the lowest electronegativity is cesium, which has a value of 0.79. Since the conceptual opposite of electronegativity is electropositivity, you could also say that the most electropositive element is cesium. The transition metals don’t vary a whole lot, either across the chart or up and down a group. The electronegativity values for the transition metals don’t vary much because their metallic properties influence how they attract electrons.
Specific Examples of Electronegativity:
- Strontium – Strontium is an alkaline earth metal with atomic number 38 and symbol Sr. It is found in Group 2 on the periodic table. Strontium was frequently used to made glass for cathode ray tube television, though as CRTs fall out of favor use of strontium is declining. It burns red when added to fireworks. Strontium has an electronegativity value of 0.95.
- Beryllium – Beryllium is a fairly rare element that occurs when cosmic rays collide with atomic nuclei. It has the atomic number 4 and its symbol is Be. Beryllium is also part of group 2 on the periodic table and as it is higher up the chart than Strontium it has an electronegativity value of 1.57. Beryllium is used to make stable but lightweight structural components for aircraft and satellites.
- Cobalt – Cobalt is a transition metal found in group 9 of the periodic table. Its atomic number is 27 and its symbol is Co. Cobalt is frequently used in the construction of lithium-ion batteries as well as a pigment for its striking blue color. It has an electronegativity value of 1.88.
- Silver – Silver is another transition metal, and it is found in group 11 of the periodic table. Its chemical symbol is Ag and it has an atomic number of 47. Silver is used to making semiconductors and also in jewelry. It has an electronegativity value of 1.93.
- Boron – Boron is a metalloid made by cosmic ray spallation, it has an atomic number of 5 and is denoted by the symbol B. Boron is often used in detergents and in semiconductors. Its also used to reinforce fiberglass. Boron is found in group 13 and it has an electronegativity value of 2.04.
- Phosphorus – Phosphorous is a reactive nonmetal found in group 15 of the periodic table. It has the atomic number 15 and is denoted by the symbol P. Phosphorous is used in fertilizers and matches. It has an electronegativity value of 2.19. Note that its presence in group 15, period 3 of the periodic table corresponds to its higher electronegativity than the elements mentioned so far.
- Hydrogen – Hydrogen is the element that the electronegativity of other elements is based around. It has an electronegativity value of 2.20 and can be found in group 1, period 1. It has the atomic number 1 and is represented by the symbol H. Hydrogen is the most abundant element in the entire universe and is used for all sorts of industrial processes such as cooling power stations and stabilizing parts of semiconductors.









