The formal chemical charge of Ammonia (NH3) is zero, it doesn’t actually have a chemical charge. While it’s easy to just say that, it’s important to place that answer in context and understand what it means in chemistry. It’s also important to know how to calculate the charge of an atom.
Let’s explore how to calculate formal charge and what it means.
What Is A Charge Number?
One of the most important factors in determining how chemicals react with one another is the electron bonds that exist between atoms. Electrons orbit the nucleus of an atom and they are negatively charged. Every atom’s electron shell has a defined number of electrons, and the atom tries to maintain this number of electrons even if doing so means that the net charge of the atom will be unbalanced. The atom also has protons, which have the same individual electric charge.
An atom’s charge is usually expressed as a number to the right of the element’s symbol: NA1+. The 1+ that represents the charge of the atom is also called the charge number.
Atoms are made up of multiple shells, and each shell has a slightly different number of electrons. The innermost shell usually has two electrons, the second shell can hold eight, and the third shell can hold up to 18 electrons. The electrons that make up the outer shell of the atom are collectively referred to as the valence shell. The valence shell is where the chemical reactions between atoms take place. An atom’s charge number reflects the fact that the valence shell of an atom will usually have a full set of electrons, whether or not the atom must gain or lose electrons to make this happen.
The Periodic Table Of The Elements
The periodic table of elements groups elements by the number of electrons that the element’s atoms have available for use in chemical reactions.
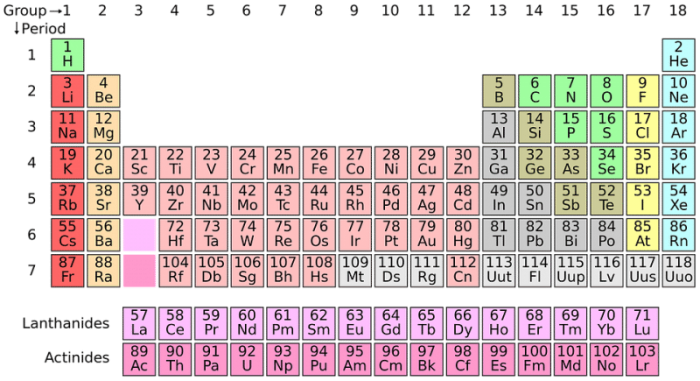
Photo: Geralt via Pixabay
Elements found at the left-most portion of the periodic table, columns I and II, have valence shells that are effectively empty. They only have one or two electrons and are mainly metals – alkali metals and alkaline earth metals. The exception to this is hydrogen, which is a diatomic nonmetal. Columns three through seven on the periodic table are nonmetals, and they have three and four electrons for reactions respectively. There are even elements that can’t react with other elements. These are called the noble gases, and they already have full valence shells. They include xenon and radon.
“I will roar argon into chlorine, xenon into fluorine, all the noble gases into reactive ones; my lament will terrify even the stars.” — Jessica Stern
An element’s charge number can be used to figure out the kind of charge an atom will possess when it reacts with other atoms during a chemical reaction. Depending on if the atom loses or gains electrons, it may develop a positive or negative charge. As an example, let’s look at an interaction between chlorine and sodium. An atom of chlorine would gain one electron upon reacting with sodium, while the sodium would lose the electron. This would make the atoms: Cl1+ and Na1-, respectively.
Note that in their unaltered form, all elements have a charge number of zero. This is because they haven’t reacted with another atom and thus haven’t lost or gained any electrons.
The Properties Of Ammonia
Now we can look at the chemical structure of ammonia and begin to understand why it doesn’t have a charge.
Ammonia is a compound made out of both hydrogen and nitrogen atoms. It’s a colorless gas that has a notable pungent smell. It can be hazardous and caustic in concentrated forms and is frequently used in cleaning solutions and fertilizers.
Ammonia’s exact chemical formula is NH3. This means that it’s made out of one nitrogen atom and three hydrogen atoms, arrayed in a pyramidal shape. Nitrogen has a lone pair of electrons, which are capable of donating an atom to hydrogen (H+). The bond isn’t a full ionic bond, it’s a covalent bond. This means that the lone pair is the negative end of a polar molecule. As such the nitrogen pair has a partial negative charge that’s balanced out by the partial positive charge at the other end of the molecule.
The lone pair of electrons can be split when the NH3 atom accepts a hydrogen proton, becoming NH4+ (ammonium). Yet until this happens, the presence of the lone pair makes an atom of NH3 a neutral molecule with a zero charge. To see why this is the case, we can calculate the formal charge of the atom.
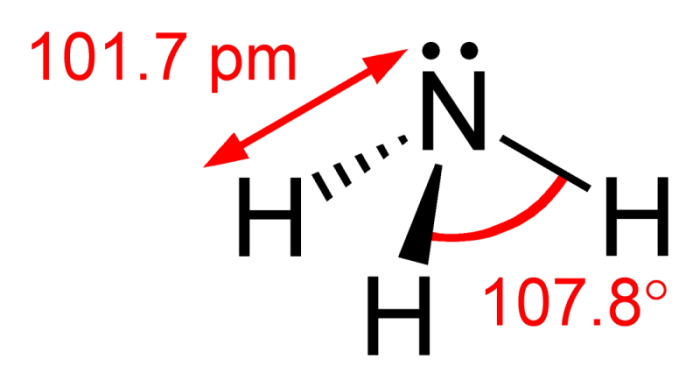
Photo: By Ben Mills – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=6628922
Calculating A Formal Charge
Calculating the formal charge of an atom is a method of electron accounting. When calculating the formal charge of an atom, it’s assumed that the electrons in a chemical bond are equally distributed across the atoms.
The equation for determining the formal charge of an atom is as follows:
- Formal Charge = [the number of the valence electrons in the atom] – [(the number of non-bonded electrons) + (the number of bonds)].
Alternatively, this formula makes the relationship between the bonding electrons and electrons in lone pairs a bit more explicit:
- Formal Charge = [number of valence electrons] – [(number of electrons in lone pairs) + (half the number of bonding electrons)]
Whichever equation you choose to use, you can use the common element charges to carry out the equation. Consulting a table of Common Element Charges reveals to us that hydrogen has a charge of +1 while nitrogen has a charge of -3.
Carrying out the operation gives us the total charge of ammonia:
Total charge = 5 (number of valence electrons) – [3 (bonds to hydrogen) + 2 (lone electrons) = 0
Therefore, an atom of ammonia has zero overall charge — a neutral charge.
Note that the values given for the common element charges only reflect the most common arrangements of the electron shell. The most common arrangements are those which have the most stability. While these stable arrangements are the most likely, other charges may show up. The aforementioned noble gases are the only atoms that don’t have variations of the electrons in their valence shell, as you can already find eight electrons in their outer shell.









