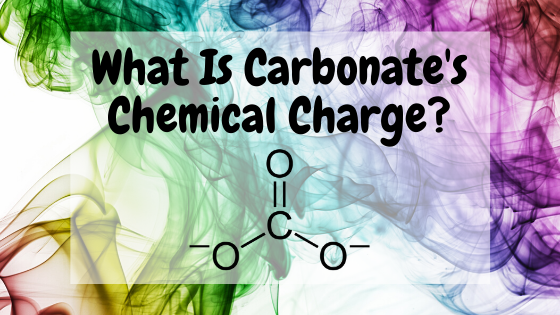
The substance with the chemical formula CO3 goes by the name carbonate. Carbonate is made of 1 atom of carbon and 3 atoms of oxygen and has an electric charge of −2. This negative charge means that a single ion of carbonate has 2 more electrons than protons.
Carbonate is a flexible polyatomic ion and is characterized by its tendency to form salt compounds with alkali and alkali earth metals. Carbonate compounds are the main component of several types of sedimentary rock, the most well known being limestone which is composed mainly of calcium carbonate (CaCO3). Carbonate compounds also make up the shells of mollusks and coral, as well as in cleaning agents like sodium carbonate (Na2CO3) and potassium carbonate (K2CO3). Carbonate compounds are also found in the human body where they are used as a buffer to regulate pH levels in the blood.
“Often referred to as osteoporosis of the ocean, [ocean acidification] prevents shell building creatures such as lobster, oyster, crab, shrimp, and coral from extracting the calcium carbonate from the water that they need to build their shells and are thus unable to survive.” — Philippe Cousteau, Jr.
Carbonate is a polyatomic ion, in that it is an ion made from 2 or more atoms. First, let’s look at the general concept of an ion and work up towards the more complex idea of a polyatomic ion.
What Is An Ion?
In a nutshell, an ion is an atom that has unequal amounts of protons and electrons. All atoms are composed of three kinds of particles, protons, neutrons, and electrons. Protons and neutrons exist bunched together in the nucleus of the atom while electrons exist in orbital shells surrounding the nucleus. each particle has an associated electric charge. Protons have a charge of +1 and electrons have a charge of −1. Neutrons have a neutral electric charge of 0.
In a normal atom, there exist equal amounts of protons and electrons. In such atoms, the positive charges of the protons and the negative charges of the electrons are exactly equal and opposite, so the charges cancel out and the atom is overall electrically neutral. This is not always the case though. Atoms can gain or lose electrons, and so take on an overall negative or positive charge. Atoms with non-zero electric charges are called ions.
There are two major kinds of ions. Positively charged atoms are called cations. Cations form when an atom loses electrons. There are now more protons than electrons so the atom has an overall positive charge. Negatively charged ions are called anions and are formed when an atom gains electrons. There are now more electrons than protons so the atom has an overall negative charge.
“By allowing the positive ions to pass through an electric field and thus giving them a certain velocity, it is possible to distinguish them from the neutral, stationary atoms.” — Johannes Stark
Take sodium (Na) as an example. A sodium atom has 11 protons and 11 electrons. Sodium has a relatively low ionization energy, meaning that its electrons can be removed easily. As such, sodium has a tendency to lose electrons and form a positive cation. The electric charge of an ion is normally written as a superscript number next to the chemical symbol. In the case of sodium, sodium normally loses 1 electron and so forms an ion with a charge of +1, written Na+. Fluorine (F), on the other hand, has a high electronegativity and easily picks up extra electrons. An atom of fluorine will pick up an extra electron to fill its outer shell and makes an ion with a charge of −1, written F−
Ions form bonds via the strong electrostatic attraction between positive and negative ions. In the case of sodium chloride (NaCl), a sodium cation will bond with a chlorine anion like so:
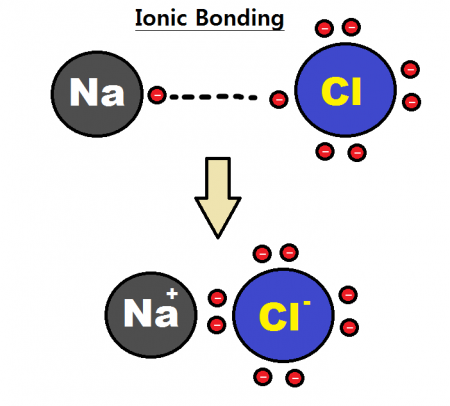
An ionic bond between a sodium cation and a chloride anion. Credit: Rhannosh via WikiCommons CC BY-SA 3.0
Ionic bonds tend to be stronger than covalent bonds due to the stronger electrostatic interaction between ions. Ionic compounds normally are brittle, have high melting/boiling points, and dissolve easily in polar solvents.
Polyatomic Ions
Polyatomic ions, as the name would imply, are ions that are made out of multiple atoms. In other words, a polyatomic ion is just a molecule that has an unequal amount of protons and electrons. Like monatomic ions, polyatomic ions have an overall positive or negative charge.
Take ammonium (NH4+) for example. Ammonium is a polyatomic ion made out of a single nitrogen atom and 4 hydrogen atoms. Ammonium has a total of 9 protons (5 for the nitrogen and 1 for each hydrogen) but only 8 electrons. So, ammonium has an overall charge of +1. Ammonium can be formed from the protonation (adding a proton) to ammonia (NH3). The addition of an extra proton gives the entire molecule a net positive charge.
Polyatomic ions are most commonly seen in the context of acid-base chemical reactions. Acidic solutions are formed by dissociating hydrogen atoms from a substance. This process results in free protons (H+ ions) and the corresponding polyatomic conjugate base pair. For instance, sulfuric acid (H2SO4) will dissociate in a solvent to make 2 H+ ions and its conjugate base, a sulfate polyatomic ion SO42−.
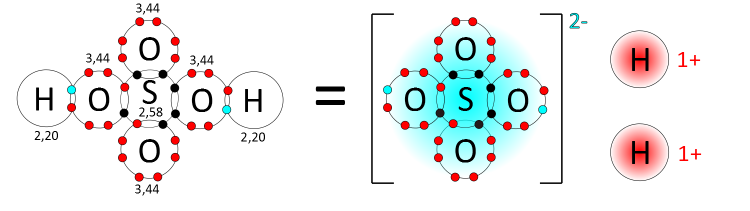
The dissociation of sulfuric acid into hydrogen cations and a sulfate anion. Credit: Riccardo Rovinetti via WikiCommons CC BY-SA 3.0. Image cropped.
Notice how the sulfate ion keeps ahold of the two electrons originally shared by the hydrogen atoms. The addition of the two electrons from the dissociated hydrogen atoms gives the sulfate ion its overall negative charge of −2.
Ions VS Polarity
Ions and polar molecules are not the same things. Polar molecules are molecules that have an electric dipole due to the uneven spatial distribution of atoms. Ions are atoms that have unequal amounts of electrons and protons. Polar compounds contain covalent bonds, ionic compounds do not. Additionally, polar molecules have partial electric charges while monatomic and polyatomic ions have integer electric charges.
Carbonate As a Polyatomic Ion
Carbonate is the simplest kind of oxocarbon ion. it is made out of 3 oxygen atoms bonded to a central carbon atom and has a symmetrical trigonal planar geometry. Carbonate has a molar mass of about 60 g/mol. It is the conjugate base of carbonic acid (H2CO3) and can be made via the dissociation of carbonic acid in a solvent.
The atomic structure of a carbonate ion cannot be represented by a single Lewis structure. Common sense would indicate that a carbonate atom consists of a central carbon atom sharing two single bonds to negative oxygens and a double bond to a neutral oxygen. Empirical observation indicates that the ion is completely symmetrical and each bond and oxygen atom is equivalent. Thus, carbonate is normally represented by a resonance Lewis structure:

The resonance structure of carbonate. Credit: WikiCommons CC0 1.0
The actual electron structure of a carbonate ion is understood to be some average of these three separate figures.
Carbonate ions are electrically negative, and so have a strong tendency to form ionic bonds with positively charged cations. The resulting substance is generally referred to as a carbonate salt. In general, carbonate ions form salts with group 1 and 2 alkali and alkaline earth metals. Alkali and alkaline earth metals, like sodium, potassium, calcium, and magnesium, tend to form positive cations so they readily bond with negative carbonate anions. One of the most common carbonate salts is calcium carbonate (CaCO3). Calcium carbonate is a salt formed by an ionic bond between a calcium cation (Ca2+) and a carbonate anion. Other common carbonate salts include potassium carbonate (K2CO3), magnesium carbonate (MgCO3) and sodium carbonate (Na2CO3).
Occurrences of Carbonate
Calcium carbonate is a major constituent of most kinds of sedimentary rock. Limestone, for example, is made of mainly calcium carbonate. Limestone can be dissolved by water because of its ionic composition. Dissolving limestone in water gives calcium cations and carbonate anions. The deposition of carbonate salts by mineralized water is the main mechanism behind the formation of stalactites and stalagmites in caves.

Stalactites are formed from carbonate compounds dissolved in water. Credit: Pixabay CC0 1.0
Carbonate is also an important biological substance. Most obviously, carbonate compounds are excreted by the human body to regulate internal pH levels. For example, when blood pH gets too low, meaning that the blood is acidic and has a high concentration of hydrogen ions, the body produces carbonate ions. The carbonate ions suck up the excess protons which raise the pH of the blood back to normal levels. When blood pH is too high, the kidneys excrete bicarbonate ions (HCO3−), which dissociate and introduce more hydrogen ions into the blood. The same mechanism is behind the use of carbonate compounds in antacids. Carbonate ions react and neutralize gastric acid, which eases symptoms of acid reflux and indigestion.
“Indigestion is charged by God with enforcing morality on the stomach.” — Victor Hugo
Carbonate compounds also play an important part in the formation of atmospheric carbon dioxide. Many marine organisms utilize carbonate buffer systems to regulate their internal pH levels. They exude these carbonate compounds which in turn are converted into carbon dioxide and released into the atmosphere from Earth’s oceans. Carbonate systems in the oceans are one of the main natural producers of atmospheric carbon dioxide. Increasing ocean temperatures can result in the formation of more carbon dioxide from carbonate compounds dissolved in the ocean, leading to higher concentrations of carbon dioxide in the atmosphere.
In the context of organic chemistry, functional groups that consist of a single carbon and 3 oxygens are often called carbonates. Though technically not polyatomic ions, carbonate functional groups retain many of the properties of their freely existing ionic cousins, including their solvent properties.









