Constructing proteins is busy work. Every second of the day, your bodies cells are making proteins based on information stored in your DNA. The sequence of nucleotide bases in DNA encodes the structure of different proteins. This information is “copied” in the form of mRNA during a process called transcription. The information stored in the mRNA is later “read” in a process called translation, and the encoded proteins are constructed. This entire sequence of going from DNA to mRNA to protein is called gene expression.
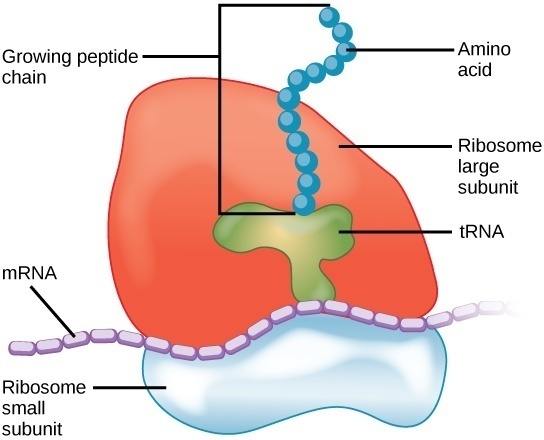
Translation is a complex process and requires some specialized molecular machinery. Ribosomes are intracellular structures that serve as the site of RNA translation. Ribosomes are the specific locations in the cell where proteins are actually constructed. The primary function of ribosomes is to construct proteins from information encoded in mRNA. Ribosomes do this by organizing the components of translation and catalyzing the reaction that binds amino acids into larger polypeptide chains. Ribosomes contain two distinct parts: a smaller subunit that reads the information in RNA, and a larger subunit that catalyzes the reaction that binds amino acids into a polypeptide chain.
Strictly speaking, ribosomes are NOT organelles. Ribosomes are not organelles because they are not membrane-enclosed units; they are free-standing particles. Additionally, ribosomes are present in prokaryotes, and prokaryotes do not have organelles. In eukaryotes, ribosomes tend to occupy the rough endoplasmic reticulum or they are free standing in the cytosol.
Ribosome Structure
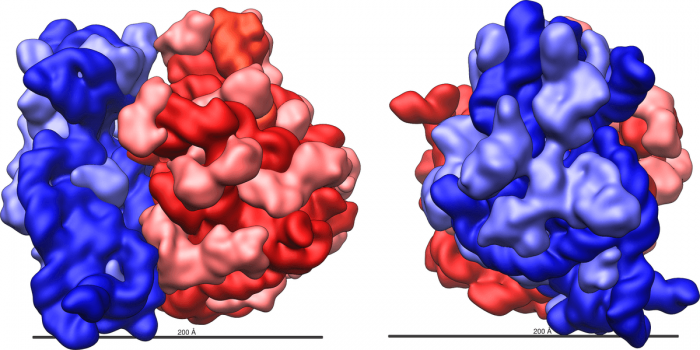
Ribosomes have 2 major substructures. Credit: Vossman via WikiCommons CC BY-SA 3.0
Ribosomes are complex particles made out of special ribosomal RNA (rRNA) and various proteins. The number of proteins varies between species. The actual physical structure of ribosomes is extraordinarily complex and consists of a deep intertwining web of RNA molecules and proteins. These molecules and proteins are arranged into two distinct ribosomal subunits of different size, known as the small and large subunit. The small and large subunit work together to synthesize proteins. The small subunits read the information stored in RNA and the large subunit organizes and catalyzes the reaction that binds amino acids together into a polypeptide chain.
Eukaryotic ribosomes are in general 20-30 nm in diameter and have an rRNA to ribosomal protein ratio of about 1. Prokaryotes tend to have more proteins than rRNA in their ribosomes, about a 65:35 ratio. Otherwise, the structure of eukaryote and prokaryote ribosomes are similar. Most kinds of ribosomes share a core structure consisting of tightly coiled and crisscrossing loops. It seems that the bulk of ribosome functioning is due to the activity of the rRNA while the proteins help stabilize the structure and catalyze reactions.
The exact number of ribosomes per cell differs depending on the tissue and cell type. In general, the more proteins that a given set of cells produces, the more ribosome on average those cells will have. Cells that are required to produce lots of protein products have a lot of ribosomes. For instance, the pancreas synthesizes a lot of enzymes for digestion, so pancreatic cells tend to have a large number of ribosomes.
Ribosome Function
Ribosomes are the main site of protein translation and biosynthesis. Ribosomes do this by pairing the codons in the mRNA strand with the appropriate tRNA anticodon. Amino acids are encoded in the form of codons, triplets of nitrogenous bases in mRNA. When mRNA is read by a ribosome, the ribosomes matches the mRNA codon with the appropriate tRNA anticodon. The tRNA carries with it the amino acid that the mRNA codon encodes for.
After transcription, there exists a strand of complete mRNA ready to have its information read. The two ribosomal subunits enclose a strand of mRNA, almost like the two buns of a hamburger. The smaller subunit attaches to the mRNA strand and begins to “walk” along its length, looking for the start codon (AUG). The large subunit contains 3 main slots for tRNA molecules, dubbed the A, P, and E sites.
As the small and large subunits walk along the length of the mRNA strand codon-by-codon, tRNA containing the appropriate anticodon and amino acid fit into the slots of the large subunit. The large subunit the catalyzes the hydrolysis reaction that joins the amino group of one amino acid to the carboxyl group of another. This process continues down the mRNA strand until the small subunit reads a stop codon and halts translation. The recognition of a stop codon initiates the activity of release factors which disassociate the ribosomal subunits, freeing the polypeptide chain.
Imagine mRNA as a strand of tape with instructions on it. During translation, the mRNA tape is fed into the ribosomal machine. The machine reads the tape until it sees the instruction to begin translation (AUG codon). The ribosomal subunits go down the strand carry out the instructions, piecing together the parts in the order specified by the mRNA. Once the ribosome machine reads a stop codon, it stops attaching parts and detaches the constructed chain. The almost-complete protein is then transferred elsewhere in the cell for some last minute post-translation modifications.
Most proteins specified by the human genome take about 1 minute to translate from RNA. The longer the protein, the longer time it takes for ribosomes to make. For example, titin is a protein consisting of a sequence of over 30,000 amino acids. One unit of titin takes approximately 1 hour to translate. In general, ribosomal subunits are reusable. As soon as one protein is disconnected, another mRNA strand enters the mix and protein translation begins again.
How Are Ribosomes Made?
Ribosomes are essential for the construction of proteins, but where do ribosomes themselves come from? In eukaryotes, ribosomes are synthesized by the cell in the cytoplasm, near the nucleus. Although it might sound paradoxical, ribosomes themselves are constructed by other ribosomes. Some chromosomes have sequences that encode for ribosomal RNA and ribosomal proteins. These rRNA strands and r-proteins are themselves constructed by ribosomes via translation. The rRNA and r-proteins then conglomerate to form the ribosomal subunits and are shuttled out of the nucleolus into the cytoplasm to do their job.
Because ribosomes are so complex, a single error in construction can compromise the functioning of the entire complex. As such, the body has extensive surveillance mechanisms in place to detect non-functional rRNA strands and non-functional mature ribosomes. At this time, it is not fully understood how exactly the body checks for all the possible errors in ribosomal structure.
Evolutionarily speaking, ribosomes are very old as they seem to have been present in the first forms of life to exist on Earth. The exact story behind the evolution of ribosomes is not known. Some have theorized that ribosomes evolved out of self-replicating RNA molecules that only later gained the ability to synthesize proteins once amino acids became common. Ribosomes could then trace their origin back to a time before the emergence of DNA and protein-based life, a hypothetical time period dubbed the “RNA-world” by evolutionary biologists.
Ribosomopathy
Disease involving abnormalities in the structure or functioning of ribosomes are called ribosomopathies. One example is Treacher-Collins syndrome, a rare genetic condition characterized by facial deformities. Treacher-Collin ssyndrome is caused by mutations in three specific gene clusters that encode for proteins that play a role in the early development of the face. These mutations cause decreased production of rRNA and ribosomal units, which can lead to the premature death of cell involved in the development of facial bones and tissue. The deformed facial bones can cause breathing and hearing problems.
Viruses and Ribosomes
Many known viruses are capable of taking over normal ribosomes to make their own viral proteins. Viruses are microscopic quasi-organisms that infect host cells and highjack their cellular machinery to reproduce. Viruses, strictly speaking, are not considered living because they cannot reproduce on their own. Instead, viruses use the host cells machinery to reproduce and assemble themselves inside the cell. Viruses are constructed, in part, by proteins so they use host cell ribosomes to make those proteins.
Viruses will attach themselves to healthy cells and inject their genetic material inside them. The injected DNA or RNA takes over the cell’s functioning and begins to construct the proteins required to assemble new viruses. Because viruses do not have any cells or organelles, they cannot produce these proteins on their own. Viral DNA is fed into the host cell’s ribosomes to construct the proteins required for viral replication. The virus continues to replicate and assemble until the cell bursts and dies.
To sum up, ribosomes are intracellular units that assist in the construction of proteins during RNA translation. Ribosomes help make proteins by organizing the mRNA, providing sites for amino acid-carrying tRNA to bond the mRNA, and catalyzing the hydrolysis reactions that link amino acids into a polypeptide chain. All living organisms, eukaryotes and prokaryotes, have ribosomes of some kind.
Ribosomes themselves are made of a special kind of ribosomal RNA and ribosomal proteins, existing together in a complex tangle. Ribosomes are further divided into two main parts; the smaller subunit that reads the information encoded in mRNA, and a larger subunit that organizes tRNA and binds amino acids to each other.