
You should never mix bleach and ammonia together because the end result is a dangerous/deadly combination of toxic vapors. The chemical reaction releases toxic vapors called chloramines, and these chloramines can potentially react with other chemicals to form a toxic, flammable substance known as hydrazine. Chloramines are respiratory irritants, things that irritate the lungs and respiratory system, making breathing difficult. Hydrazine, in addition to irritating the respiratory system, can cause various other symptoms such as seizures, nausea, edema, and headaches.
“The right cleaning product can make grease clean up a snap. A lot of people think bleach does the trick, but the truth is that there are a lot of great alternatives that are easy to use and kill the germs just as well, but are a lot less harsh than bleach.” — Kevin Boyd
Mixing ammonia and bleach together produces chloramine vapors, but so can mixing cleaning chemicals together in general. Using chlorine bleach to disinfect surfaces or substances containing certain organic matter, such as pond water can also produce chloramines.
So that you understand why the two chemicals should never be mixed together, let’s take a close look at the individual chemicals involved in the reactions and the toxic products of the reactions.
Facts About Bleach

Photo: By Adina Firestone – IMG_7066 (1), CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=38047217
Bleach is the name given to any industrial commercial cleaning chemical used to remove stains or white clothes. Bleach is made out of diluted sodium hypochlorite, which has the formula NaOCl or NaClO. The sodium hypochlorite quickly decomposes, and as it does so it liberates molecules of chlorine, which is the primary chemical reaction that gives bleach its cleaning property.
Chlorine bleach is a powerful disinfectant, with the reaction being an oxidization reaction that degrades microbial cells. The oxidation process is also responsible for the removal of pigments, which gives bleach its whitening properties. The sodium hypochlorite breaks down the bonds within the chromophore of a molecule. Note that this only applies to chlorine-based bleach, and peroxide bleach is not an effective disinfectant.
A strong chlorine odor is notable when using bleach, but the use of bleach actually typically produces saltwater as a byproduct rather than chlorine gas. Chlorine-based bleaches tend to be more effective when they are diluted as opposed to being used at full strength, and for this reason, it is usually recommended to combine nine parts water to one part bleach. Bleach is corrosive in nature and because of this, if you’re cleaning metal surfaces with bleach you should clean the surface with pure water afterward.
Bleach releases toxic chlorine vapors when it reacts with certain other chemicals such as vinegar, alcohol, acetone, and of course ammonia. In general, you should avoid mixing bleach with other cleaners. Be sure to follow the directions on the label at all times.
“We dream of having a clean house, but who dreams of actually doing the cleaning?” — Marcus Buckingham
While a dangerous chemical known as dioxin (an environmentally persistent and extremely toxic form of organic pollution) is occasionally released when bleach is used on paper products and wood pulp, household bleach does not form it dioxin because in order for the chemical to form gaseous chlorine must be present.
Facts About Ammonia
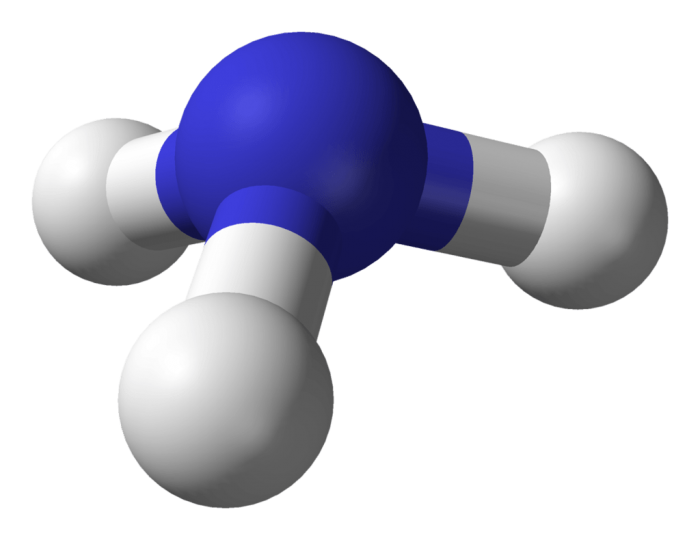
PHoto: Photo: By Ben Mills – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=3958453
Ammonia is a chemical compound that has the formula NH3, being made out of one nitrogen atom and three hydrogen atoms. It is colorless, though it has a distinctive, pungent, unpleasant odor. Ammonia is frequently produced as a nitrogenous waste product, and while it is important for the production of certain fertilizers and pharmaceutical products, as well as an ingredient in many commercial cleaning products, the substance is also hazardous. Ammonia can be toxic in high concentrations, as well as caustic. For these reasons, laboratories and other facilities which deal with ammonia must abide by strict storage regulations.
Around the globe, almost 90% of ammonia is used in the production of fertilizers, used to create solutions or salts. These ammonia-based fertilizers produce higher yields of crops like wheat. Ammonia is also often used to kill E. coli and sterilize some cuts of beef.
Ammonia is a common ingredient in many general-purpose cleaners. The type of ammonia found in cleaning solutions is ammonium hydroxide, ammonia dissolved into a solution of water. Ammonia is used in cleaners because it produces a fairly streak-free shine compared to other cleaning chemicals, and is therefore frequently used to clean things like glass and stainless steel. Ammonia-based cleaners are also good at cutting through grease and grime, and as a result, they are frequently used to clean ovens, microwaves, and other surfaces with baked-on grease or grime.
Chemicals Emitted From Combinations Of Ammonia And Bleach
Chemicals emitted by combining ammonia and bleach include the following:
- Nh3 – ammonia, though it may be somewhat surprising that combining ammonia and bleach can release ammonia itself, it can happen.
- HCL – hydrochloric acid.
- NaOCl – sodium hypochlorite, which is bleach and can, as described above come in different forms.
- Ch – chlorine, or cl2 – chlorine gas.
- Nh2Cl – another chlorine related product.
- N2H4 – hydrazine, as mentioned previously is a possible result of the combination of chloramines.
- NaCl is one of the few nontoxic potential results of the combination of bleach and chloride, as it is just table salt.
- H20 – water, another nontoxic byproduct.
Possible Chemical Reactions Produced By Combining Ammonia And Bleach
When bleach decomposes it creates HCl (hydrochloric acid), and this acid forms dangerous chloramine fumes which when combined with ammonia. The actual process that gives rise to these fumes can be visualized like this:
- HCl is created when bleach decomposes:
- NaOCl (sodium hypochlorite/bleach), which in turn becomes NaOH + HOCl.
- The hypochlorous acid (HOCl) then becomes HCl (hydrogen chloride) + O.
- Afterward, the chlorine gas and ammonia interact and perform chloramine, which materializes as a toxic vapor.
- NaOCl and 2HCl end up becoming chlorine gas, salt, and water: Cl2 + NaCl + H2O.
- The ammonia – 2NH3 then combines with the Cl2 to form 2NH2Cl (chloramine).
Excess ammonia may even form liquid hydrazine which is both toxic and explosive. Though unrefined hydrazine won’t typically explode, the compound is unstable and can quickly boil, spraying out toxic liquids into the surrounding area. This reaction can be summarized like this:
2NH3 (ammonia) + NaOCl (bleach) → N2H4 (hydrazine) + NaCl (salt) + H2O (water)
First Aid For Ammonia And Bleach Exposure
While it is important to avoid mixing ammonia and bleach together, accidents happen. If you have accidentally been exposed to and ammonia and bleach mixture, withdraw from the area immediately and seek fresh air, then look for medical attention. The vapors that arise from the history of ammonium bleach will irritate your mucous membrane and eyes, but inhaling the toxic fumes is the greatest threat to your health.
“The dose makes the poison.” — Paracelsus
First Aid Step 1: Immediately leave the area where the chemicals were mixed together and the feelings were produced. It is important to ensure your safety, even if there are other people in the area, as you cannot contact emergency services or medical professionals if the feelings overwhelm you.
First Aid Step 2: Contact 911 immediately, if someone has been overcome by fumes. If less severe exposure has occurred, contact poison control, who will be able to provide you with information on treating symptoms of exposure and handling the cleanup of the chemicals.
First Aid Step 3: If a person is suffering from exposure to and ammonia and bleach mixture, and is unconscious, the person should be moved to an area of fresh air if it can be done safely. Emergency services should be contacted immediately.
First Aid Step 4: Poison control should be contacted for instructions regarding the proper cleanup of the chemicals as well as safe disposal.
Other Chemical Combinations To Avoid
Avoid combining multiple types of drain cleaners together. Drain cleaners have powerful formulas that can react in unpredictable ways. Mixing different drain cleaners together could even lead to an explosive result, and therefore it is recommended that if one drain cleaner does not work, another product is not tried immediately afterward. In addition, toilet cleaners or drain cleaners should never be mixed with bleach as it will create chlorine gas. If one drain cleaner does not work, call a plumber.
Be careful about using hydrogen peroxide and vinegar as well. Alternating the two substances to clean surfaces, as long as the service is wiped down in between sprays, should be safe. However, the two products should not be mixed in the same container as it can potentially create peracetic acid, which irritates the respiratory system, skin, and eyes.
Similar to combining bleach and ammonia together, bleach and vinegar can produce chlorine gas when combined and therefore the two should not be used together. Chlorine gas causes difficulty breathing and irritates the mucous membrane. One should also avoid combining bleach and rubbing alcohol together, as it can potentially create chloroform, a toxic and irritating combination. In fact, in general, bleach shouldn’t be mixed with anything other than pure water.
While nothing extremely dangerous will happen if mixing vinegar and baking soda, mixing the two products together will cause a lot of foam and the two chemicals essentially neutralize one another and the resulting solution is fairly worthless for cleaning. Combining the two chemicals together and storing them in the same bottle could lead to an explosion.









