
Osmosis refers to the movement of molecules across a selectively permeable membrane. The process of osmosis has molecules spread out across a membrane gradient until the concentrations of the molecules are roughly equivalent on both sides of the membrane. Osmosis is a critical process in biological organisms, helping control levels of molecules like lipids, nitrogen, carbon dioxide, water, and oxygen. Osmosis is the primary method through which water is transported in and out of cells, and this function is necessary for cells to maintain homeostasis.
That’s the short answer on what osmosis is, but let’s see look closer at how osmosis impacts the movement of molecules across a membrane and how this affects the function of a cell.
Definition Of Osmosis
The process of osmosis is the dispersal of solvent molecules across a semipermeable membrane, moving from an area of higher molecular concentration to an area of lower concentration, or to put that another way the molecules move from more concentrated solutions to more dilute solutions. The movement of the molecules will continue until the concentration gradient – the number of molecules dispersed throughout the area – is roughly equivalently distributed. The most common solvent moved by osmosis is water, although other solvents such as gas and other forms of liquid can sometimes undergo osmosis.
The membranes that solvent molecules move through are semi-permeable in the sense that they only let certain kinds of molecules pass through. Large, polar molecules like polysaccharides, proteins, ions, and similar molecules can’t move across the membrane. However, small non-polar molecules like nitrogen, oxygen and carbon dioxide can move across the membrane.
When there are solutions on both sides of a semi-permeable membrane, the solutes particles cannot move past the membrane by themselves. Rather, the solvent molecules move across the membrane. As the solvent molecules disperse, the system moves closer to a state of equilibrium. The more equally the molecules are dispersed the more stable the system is.
Examples Of Osmosis
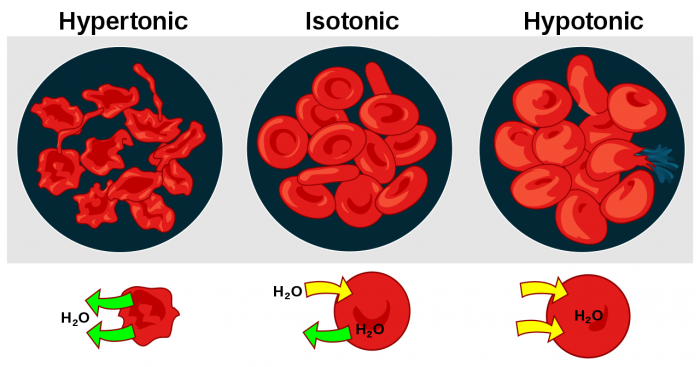
The effects of different solutions on blood cells affected by osmosis. Photo: By LadyofHats – did it myself based on [1], [2] ,[3] and [4]., Public Domain, https://commons.wikimedia.org/w/index.php?curid=1685492
Another example of osmosis is related to how minerals and salts in water are shifted around. The water itself will flow into the cells. Moving across the plasma membrane, and the osmotic process helps maintain the correct concentration of salt, glucose, and water, which is necessary to prevent cell damage. This process can be witnessed in action when looking at saltwater fish. Saltwater fish have evolved to live in bodies of water with high saline concentrations. Because of the high concentrations of salt in the water, the fish’s cell must have a lower concentration of salt. Therefore, the salt in the surrounding environment pulls water from the body of the fish and the osmotic process is regulated like this.

A Tench, a freshwater fish. Photo: By Karelj – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=14944260
By contrast, freshwater fish have to maintain their homeostasis in a slightly different way. As you might be able to guess, freshwater fish cells have salt concentrations which are higher than the surrounding environment. Thanks to osmosis, they fish don’t need to drink water because the salt in their cells absorbs the water.
Osmosis is critical for the survival of human cells and the human body at large, with osmosis regulating the proper functioning of the kidneys. Kidney cells use osmosis to pull water from the waste products of other organ systems. In fact, kidney dialysis is an example of the osmosis process. Individuals who have kidney diseases undergo kidney dialysis which pulls waste products from the blood, pulling the molecules through a dialyzing membrane. The molecules are then passed into a tank full of dialysis solution. The red blood cells will be maintained in the blood itself because they are too large to pass through the membrane, but the waste products are removed.
It is also thought that the people’s skin becoming wrinkly after being submerged in water for a long time is the result of osmosis, although recent research has challenged this idea.
Variations Of Osmosis
There are variations of osmosis such as reverse osmosis and forward osmosis. Reverse osmosis is a process that has pressure forcing solvents across membranes. In reverse osmosis, one side of the membrane retains the solute while the pure solvent is pushed through to the other side of the membrane. Reverse osmosis pushes the solvent from its default position in a region of high concentration to the low solute concentration region with excess osmotic pressure.
Forward osmosis is used to separate water out of solutions with other solutes in it. The solution of higher osmotic pressure is utilized to push the water through a semi-permeable membrane so that the “feed solution” (the solution with lower osmotic pressure) ends up becoming concentrated as the higher pressure solution dilutes. The newly diluted solution is then either sent through a secondary processing operation or used directly. Forward osmosis is frequently used for things like water treatment, desalination, and water purification.
History Of Osmosis
The process of osmosis was first documented by Jean-Antoine Nollet around 1748. While Jean-Antoine Nollet was the first person to describe the phenomenon, the actual term was coined by René Joachim Henri Dutrochet, a French physician. Dutrochet based the word osmosis on the words “exosomose” and “endosmose”. Moritz Traube developed more sophisticated techniques for measuring osmotic flow in 1867.
Diffusion
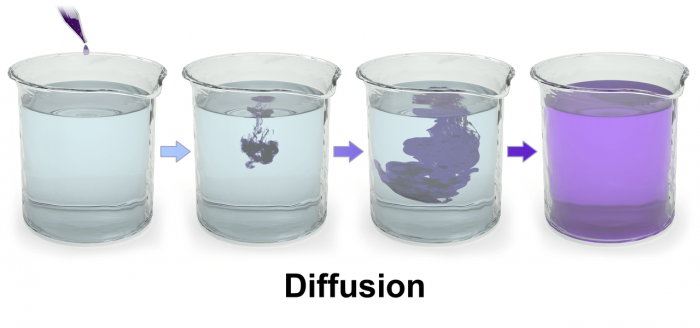
Photo: By BruceBlaus – Own work, CC BY 3.0, https://commons.wikimedia.org/w/index.php?curid=29452222
Diffusion is another method of mass transport in biology and chemistry, and while it also involves the moving of molecules it differs from osmosis in important ways.
Diffusion occurs when molecules, ions, and water leave or enter cells. Cellular diffusion happens when molecules move from an area of higher concentration to a region of lower concentration. This movement will continue to occur until both halves of the region have approximately the same amount of molecules with them until the distribution of molecules is approximately equal. Different cells can have different rates of diffusion.
There are multiple types of diffusion within biology, with two of the most common forms of diffusion being active transport and passive transport. The difference between these two different forms of fiction is that active transport uses energy to push molecules from an area of lower concentration a higher concentration, while in passive transport molecules naturally are dispersed from an area of higher concentration to a region of lower concentration.
Passive transport occurs naturally with these substances moving across a semipermeable membrane without needing to use energy to do so. The rate the substances are transported at relates to the permeability of the membrane. The membrane of a cell controls what types of substances can move through it, enabling certain substances to move through it while blocking others. The permeability rate affects how easily substances can move across the membrane, with an example being a cell wall. Plant cells have cell walls which surround the inner cell membrane and this structure has very low permeability, keeping most molecules out.
Facilitated diffusion can be thought of as a subtype of passive transport. With facilitated diffusion, special transport proteins enable molecules to move across the cellular membrane more easily. These transport proteins allow larger molecules to penetrate the cell membrane when the men were not able to do so. Molecules like glucose are transported across the membrane and diffuse through facilitated diffusion. In facilitated diffusion, a carrier protein binds itself to the molecule and the molecule is pulled through the cell membrane by this protein.
Active transport is the conceptual opposite of passive transport, moving molecules from a region of lower concentration to an area of higher concentration. Primary active transport utilizes metabolic energy to force molecules through a cellular membrane. However, there is a type of active transport referred to as secondary active transport. While cellular transportation systems are used to move molecules in this secondary form of transport, ATP is not used to do it. Instead, entropy and ion pumps are used to transport the molecules, and a difference in the chemical potential results.









