
50 years ago asthma was a rarity, and many allergies that are now commonplace were unheard of. Yet today, the CDC estimates asthma rates have increased by 28 percent since 2001. We’re feeling the cost of this downturn in health: per-person spending for healthcare rose by 4.2 percent in 2017 alone, with rising diabetes rates being the biggest contributor.
Meanwhile, the healthcare community has been desperately looking for clues as to why the rate of these conditions has seen such a steep increase. While environment and diet are certainly factors, there is growing evidence that something more simple may be at play. Interestingly, colic, asthma, eczema, diabetes, allergies, and inflammatory bowel diseases (IBD) have something in common–they’ve all been shown to be connected to intestinal inflammation. And now, evidence is emerging that intestinal inflammation may be closely tied to the absence of the beneficial bacteria B. infantis in the infant gut.
Our study, recently published in Pediatric Research, shows that colonizing infants with a specific strain of Bifidobacterium, B. infantis EVC001, reduces intestinal inflammation up to 55-fold compared to controls. The timing may be critical, as an infant’s first 100 days coincides with a period of rapid growth and development, including programming of the immune system. Reducing inflammation during this window, therefore, may have both acute and long term health implications, and could play a role in going beyond relieving the symptoms to treat these problems at their source.
Reduction in key markers of inflammation
Intestinal inflammation is primarily measured by the levels of specific markers that appear in fecal samples, including cytokines, calprotectin, and endotoxin — our study showed that infants who received B. infantis EVC001 produced significantly lower levels of all of these. For example, infants fed B. infantis EVC001 showed up to a 55-fold reduction in proinflammatory cytokines–particularly IL-1β, TNFα, and IFNγ–which are associated with increased intestinal permeability, a condition that may precede the development of autoimmune disorders such as type 1 diabetes later in life.
Additionally, infants with low Bifidobacterium showed calprotectin levels similar to levels shown to cause 2x higher risk of asthma and atopic dermatitis in term infants. In contrast, higher Bifidobacterium abundance was significantly correlated with lower fecal levels of calprotectin and may serve a protective function. This is, in fact, the first time fecal calprotectin concentration has been shown to be negatively correlated with Bifidobacterium abundance.
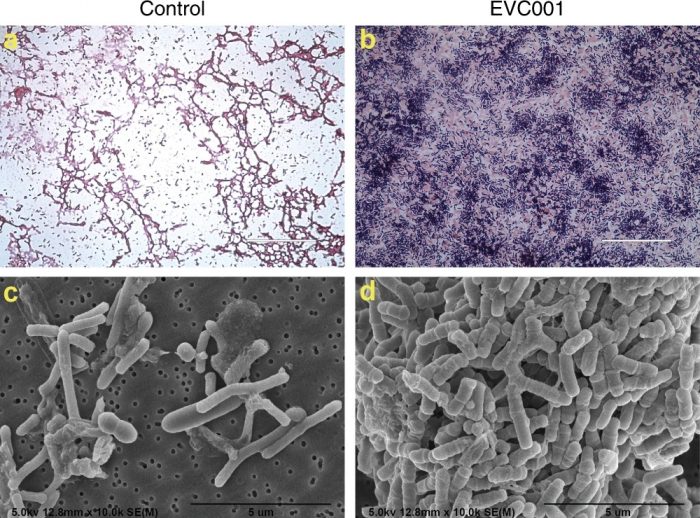
Figure 1: Microscopic analysis of the infant gut microbiome. Gram stain light microscopy (a, b) and scanning electron microscopy (c, d) micrographs of diluted fecal samples on day 40 postnatal from the control infants (a, c) and infants fed EVC001 (b, d). Scale bars: 50 µm (a, b) and 5 µm (c, d). Figure republished with permission from https://doi.org/10.1038/s41390-019-0533-2, licensed under CC-BY 4.0 http://creativecommons.org/licenses/by/4.0/
Furthermore, infants with high levels of Bifidobacterium showed a four-fold lower concentration of fecal endotoxin, which drives TLR4-based inflammation — a key factor in the onset of necrotizing enterocolitis (NEC), a common and often fatal condition among newborns.
Bifidobacterium: unique amongst its peers
Interestingly, while our study looked at the effects of 37 bacteria commonly found in the infant gut (many of which are commonly used in probiotic supplements), Bifidobacterium was the only gut bacterium found to be correlated with a significant reduction in key markers of inflammation in this study. While it has been noted by the scientific community that probiotic supplements are typically transient in the gut, the study also showed that this particular strain of B. infantis (EVC001) remains colonized well beyond the period when the infants were being fed this probiotic bacteria. The reduction in inflammation was shown to persist through the end of the full 60 days of the trial, 30 days after B. infantis EVC001 feeding discontinued, indicating an ongoing protective effect of this probiotic strain.
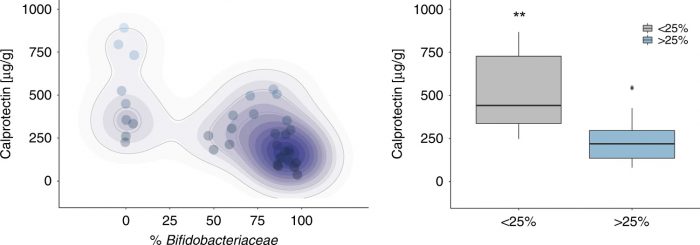
Figure 2: Fecal calprotectin levels are dependent on the abundance of Bifidobacteriaceae. Forty fecal samples from day 40 postnatal were evaluated for the concentration of fecal calprotectin and Bifidobacteriaceae abundance (****P < 0.0001; rs = −0.72; a) and subdivided based on Bifidobacteriaceae abundance < or >25% (b). The data set is representative of at least three different experiments completed in duplicate and a non-parametric Wilcoxon rank-sum test was used to determine significance with the corresponding P values adjusted and considered statistically significant if *P < 0.05. **P < 0.01. Figure republished with permission from https://doi.org/10.1038/s41390-019-0533-2, licensed under CC-BY 4.0 http://creativecommons.org/licenses/by/4.0/
Reconsidering the role of B. infantis
While B. infantis may have been traditionally thought of merely as a potentially beneficial probiotic, new research is increasingly showing us that it is much more than that. It is nothing short of an organism that co-evolved with humans to provide an important protective mechanism for infants from pathogens. However, previous studies have also shown that B. infantis has been nearly eliminated in industrialized countries by modern health practices such as antibiotics, formula feeding, and C-sections. Now that it’s missing, we are feeling the ripple effect of an infant microbiome that has been radically altered and disrupted.
To be clear, B. infantis is not a miracle cure, and by itself, it isn’t going to stem the rising tide of the aforementioned health problems we’ve been discussing. However, as a critical part of our natural defense against pathogens that disrupt immune development, getting back to our normal state — and perhaps avoiding the development of asthma, allergies, diabetes, and other autoimmune and allergic conditions — will undoubtedly involve reintroducing B. infantis to the infant microbiome.









