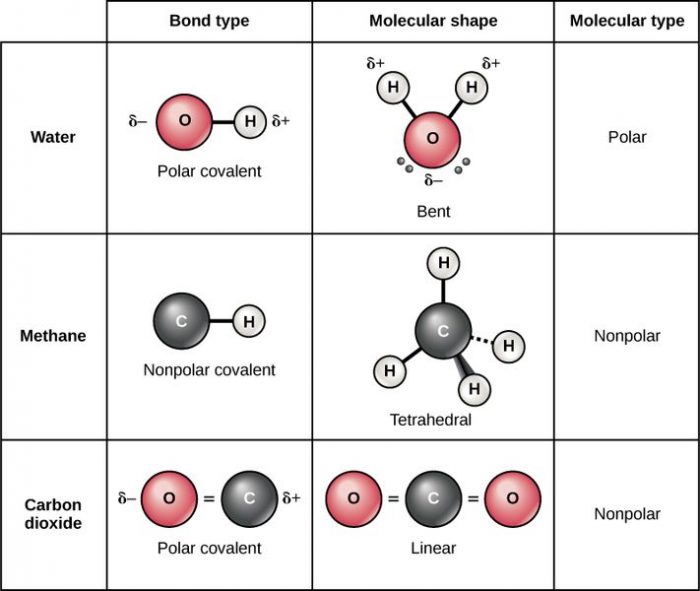
Nonpolar covalent bonds are bonds where both atoms possess the same electronegativity, and therefore the electrons in the electron bond are shared equally between them. Note that this must occur between two nonmetal atoms in order for it to be a proper nonpolar covalent bond.
That’s a quick definition of nonpolar covalent bonds, but a closer examination of what it means for a bond to be covalent and what it means for a substance to be nonpolar will help you understand nonpolar covalent bonds in greater detail.
What Is A Covalent Bond?
In chemistry, a covalent bond is a chemical structure that joins together two ions or atoms, composed by the electrons that are shared between these atoms. Covalent bonds are also sometimes referred to as molecular bonds. Covalent bonds usually happen between two atoms which are fairly close in electronegativity, and which are nonmetal in nature. That said, other chemical species can also form covalent bonds such as macromolecules and radicals. The pairs of electrons which establish a covalent bond are often called shared pairs, sometimes called bonding pairs. The effect of the shared pairs is that both atoms involved in the bond typically have a stable outer electron shell, stability similar to what is witnessed in atoms of the noble gas elements.
A chemical compound that contains atoms which form covalent bonds is referred to as a covalent compound. Covalent compounds are one of the two categories that compounds are usually grouped into. The other kind of compound is an ionic compound, and ionic compounds are composed of electrically charged molecules or atoms, thanks to an electrical imbalance caused by losing or gaining electrons. This typically occurs as a result of metal elements interacting with a nonmetal element. Examples of covalent compounds include DNA, water, and sucrose.
The two most notable types of covalent bonds are polar covalent bonds and pure/nonpolar covalent bonds. What distinguishes nonpolar covalent bonds is that their electrons are shared equally. Identical atoms, atoms which have the same on the electronegativity values have equal sharing of electrons, but the definition is occasionally stretched to cover any atoms that have an approximately equal distribution of electrons, atoms with electronegativity differences smaller than 0.4. Molecules that have such nonpolar bonds include CH4, N2, and H2.
The electronegativity of the two atoms influences how the electrons forming a bond are shared between the atoms. As the difference in electronegativity grows, the bond between electron pairs becomes more associated with one atom than the other atom. There are various thresholds that distinguish the characteristics of a bond. Bonds that have electronegativity differences from 0 to 0.3 are considered nonpolar bonds, while those that have differences ranging from 0.4 to 1.7 are classified as polar bonds. Finally, ionic bonds are those where the electronegativity difference is over 1.7.
Let’s look at some examples of covalent bonds. Covalent bonds are found in water molecules, which have the chemical formula H2O. Each hydrogen atom in the molecule shares a covalent bond with the oxygen. There are two electrons in each of the covalent bonds, one coming from the hydrogen atom and one originating from the oxygen atom. Since the electronegativity of the two atoms is relatively similar, the electrons are shared between the two atoms more or less equally.
The hydrogen molecule in H2O is made out of two hydrogen atoms themselves linked by a covalent bond. The hydrogen atom maintains a stable outer electron shell by having two electrons in this shell, and the positive charge of the hydrogen’s atomic nuclei attracts electrons to it, which keeps the molecules individual constituents held together. Phosphorus is capable of forming two different molecules when combined with chlorine. Phosphorus and chlorine can create either PCl3 or PCl5.
Both of these molecules have covalent bonds joining the phosphorus and chlorine atoms together, and in the case of PCl3 the molecule takes on of the traditional noble gas structures, where the outer electron shell is completely full. While PCl3 is a stable molecule, PCl5 also happens to be a stable molecule, so while the octet rule is a handy heuristic for determining the stability of a molecule, one should remember that covalent bonds don’t always follow this rule.
What Is Polarity?
In your day-to-day life, you are most likely to hear the term “polar” in relation to the poles at the extremes of the earth, the North and South poles. The location of these poles are at opposing points on the surface of the planet. Much like the poles found at the extreme north and south of the Earth, a battery also has poles, a negative end, and a positive end. In the context of batteries, one end of the battery has a positive charge while the opposite and has a negative charge. Much like batteries, atoms can have poles and polarity as well, even the bonds that are found between atoms can have polarity. Molecules are classified as polar when the atoms comprising the molecule are arrayed in a way that one end of the molecule has a net negative charge while the opposite and has a net positive charge.
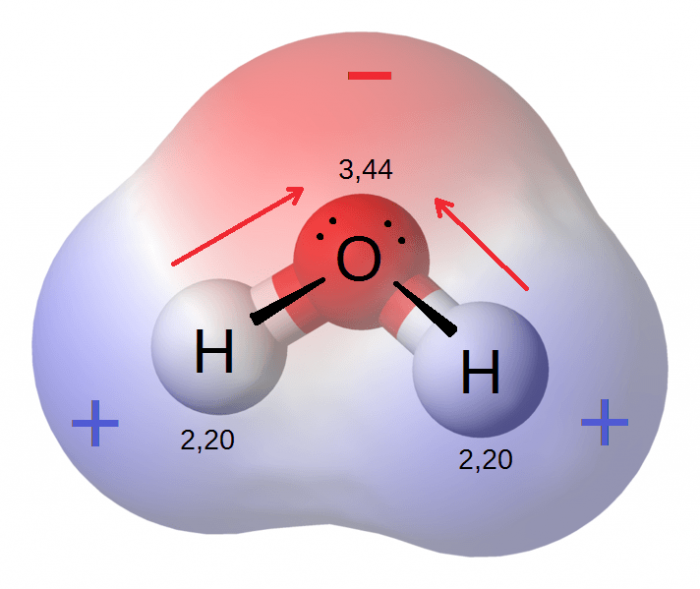
A water molecule. Blue represents positive charge areas, red negative charge areas. Photo: Riccardo Rovinetti via Wikimedia Commons, CC 3.0
Atoms which have a weak electronegativity can bond with those that have a higher electronegativity value, and when this occurs a polar molecule is created. Electrical poles are created when atoms with varying electronegativity levels join, and the polar nature of molecules created in this fashion means that they can bond with many other kinds of molecules. The fact that polar molecules have both a negative region and positive region enables them to bond with a wide variety of other chemical structures. Water is a polar molecule, and thanks to as polar nature it is able to join many other molecules and serve as the basis for life on Earth.
If polar molecules have electrical poles, you may be able to guess that nonpolar molecules lack electrical poles. Nonpolar molecules lack a noticeable charge on either half of the structure, having a more or less even distribution of electrons across the entire molecule. So to sum up, nonpolar molecules lack differences in charges on either end of the molecule, with the charges of atoms canceling each other out. Meanwhile, polar molecules are notable for the fact that their dipoles have both a net negative charge and a net positive charge.
Examples Of Polar And Nonpolar Molecules
Let’s go over some specific examples of polar and nonpolar molecules.
As mentioned, water is a polar molecule and the bonds that join the oxygen and hydrogen atoms together are distributed such that there is an equal amount of space on both halves of the oxygen-hydrogen bond, and this arrangement means that one half of the molecule has a net negative charge while the other half maintains a positive charge. Another polar molecule is ethanol, which gets its polar nature due to the fact that the oxygen atoms found within the molecule have a greater electronegativity than the atoms surrounding. The higher electronegativity potential means that these oxygen atoms attract more electrons than the hydrogen atoms, giving the – OH bonds and a total negative charge. Other polar molecules include sulfur dioxide and hydrogen sulfide.
Nonpolar molecules include molecules such as ozone, oxygen, and nitrogen. In the case of these nonpolar molecules they are comprised out of a single element, and they are referred to as homonuclear molecules. There are molecules that are homonuclear, and they include carbon dioxide, carbon tetrachloride, and methane. Substances like gasoline, toluene and acetic acid also tend to be nonpolar.

Diagram of carbon monoxide. Photo: Benjah-bmm27 via Wikimedia Commons, Public Domain
While carbon monoxide is a polar molecule, most carbon compounds are nonpolar, with carbon monoxide’s unique structure making it a polar molecule and an exception to that rule. Carbon monoxide has an electronegativity difference between the oxygen and carbon molecules great enough that the molecule has a difference in charges and is polar. Another way that carbon monoxide is unusual is that while most linear molecules are nonpolar, carbon monoxide is polar. Other nonpolar molecules include the noble gases and alkynes. The noble gases, sometimes called inert gases, are nonpolar because they are made only out of atoms of the same element, while alkynes can dissolve in water. Helium, krypton, argon, and neon are all examples of noble gases.
The electronegativity values of a molecules atom’s are something that determines whether or not a molecule is nonpolar or polar. After all, the electrons are shared equally between atoms if there is are large differences in electronegativity values, and if the values are shared unequally the molecule will have a net negative charge in one area and a net positive charge in another, making it polar. Remember that the total electronegativity, the electronegativity for all bonds, must be taken into account. One of the primary factors influencing a molecule’s polarity is the molecule’s structure/geometry. If the molecule’s charges are all orbiting a central atom, in all likelihood they are evenly distributed and the molecule will be nonpolar as a result. When predicting the polarity of a molecule both the total electronegativity and the structure of the molecule must be considered.