
Every second of each day, billions of chemical reactions are occurring in your body. These reactions extract energy from organic material to drive biological processes. In living cells, these reactions proceed extremely quickly, but when performed in the lab, they happen much slower. Why do metabolic reactions in the human body take place much quicker than the same reactions outside of the human body? The answer: enzymes.
Enzymes are biological macromolecules that accelerate chemical reactions. Metabolic reactions happen at a quicker rate in the human body due to the presence of enzymes that lower the activation energy required for those reactions to occur. Chemical entities that accelerate chemical reactions are known generally as “catalysts.” Enzymes are a specific kind of biological catalyst that makes metabolic reactions in living things occur quicker. Most enzymes are kinds of proteins, though other molecules like RNA can act as enzymes too. Enzymes are required for the body to quickly produce the large amounts of energy needed to drive biological processes. Without enzymes, the body would not be able to extract energy at a fast enough rate to be effectively used.
What Is A Catalyst?
Imagine a chemical reaction between substance A and substance B in which they bond to form one molecule AB. Depending on the circumstances (i.e. temperature, pressure, concentration, etc.) this reaction will occur at a certain speed. Say the reaction between A and B forms 3 molecules of AB per second. The reaction also requires a certain amount of energy to proceed, called the activation energy.
Catalysts work by lowering the activation energy for a given reaction. With the addition of catalysts, chemical reactions require less energy to happen, and so the reaction can proceed at a much quicker and more efficient rate. Adding an enzyme to the reaction of A and B could increase this rate to 12 AB molecules a second, for example.
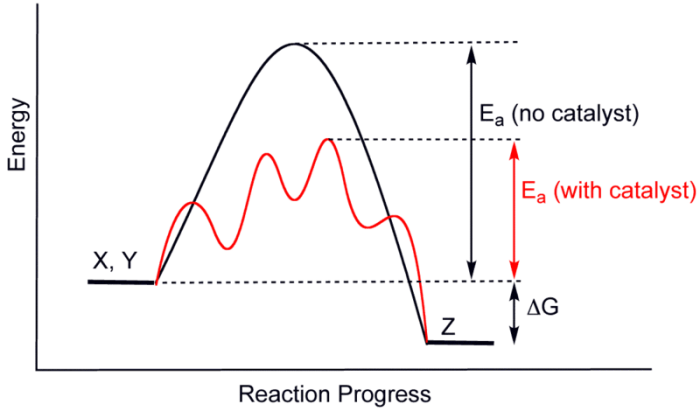
Catalysts work by lowering the activation energy necessary for a chemical reaction to occur. The above graph shows differences in activation energies with and without a catalyst. Credit: WikiCommons CC0 1,0
A catalyst is different than a reactant. Reactants are the raw materials that are eventually converted into the end products. The catalyst itself is not used up during this reaction though. Catalysts speed up a chemical reaction, but they are not consumed by the reaction. Dumping a bucket of gasoline over a campfire would speed up the combustion reaction, but the gasoline is being consumed, so the gasoline is not acting as a catalyst in this situation. Eventually, the fire would burn all of the gasoline and die out. Catalysts remain after the completion of a chemical reaction.
A simple example of a catalyst is the catalytic converter in the emission control system in your car. Catalytic converters facilitate the reaction of nitric oxide (NO) into nitrogen (N2) and oxygen (O2). The part is a container with a series of screens coated in precious metals like platinum. The metals in these screens speed up the conversion of nitric oxide into nitrogen and oxygen. The metals themselves are not consumed during the reaction, so they are acting as catalysts.
Enzymes Are Biological Catalysts
Enzymes are just a specific kind of catalyst, ones that are found in the chemical reactions in living things. Enzymes are required in living things because without them, metabolic reactions would not be able to proceed fast enough to produce the amounts of energy required by the body. Like all catalysts, enzymes in the body are not consumed during chemical reactions.
Why do living things have enzymes? Consider enzymes in the context of evolution. Living things consume food which is broken down and converted into “fuel” that the body burns to produce the organism alive. The more fuel the body burns, the more energy can be put towards those crucial biological processes. However, in the evolutionary world, resources are scarce and hard to come by. The amount of energy an organism’s metabolism can produce is constrained by its environment and its own physical makeup. Food is not unlimited, the organism has to expend energy to acquire food, living things need to remove waste products, etc. Energy is required to do all of these things, but energy in the evolutionary world is not cheap.
Enzymes are the solution to this dilemma. Enzymes allow living organisms to “optimize” chemical reactions so that energy production is maximized while energy consumption is minimized. Lowering the activation energy for metabolic reactions lets living things expend energy more frugally, while still being able to produce enough energy to stay alive. Over time, living organisms have evolved to have enzymes to maximize their odds of survival. The fact that living things developed to have enzymes at all is a testament to natural selection’s ability to produce the most elegant of solutions to the most complex of problems.
How Do Enzymes Speed Up Reactions?
The reactants that enzymes bond to are called substrates. Substrates bind to enzymes on their activation site. We know that when enzymes bind to their substrates they lower the activation energy required for chemical reactions to take place. How, exactly, do enzymes lower the activation energy though? The answer depends on the enzyme. There are numerous mechanisms that enzymes use to make reactions go faster. Enzymes can speed up reactions by spatially aligning reactants, by providing an alternate pathway for chemical reactions to occur, and by stabilizing transition states to lower their entropy change.
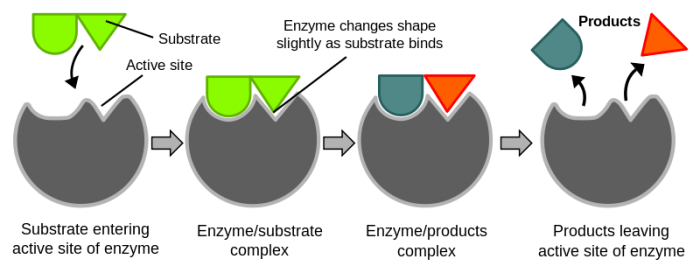
Binding to the enzymes active site changes the shape of enzymes and reactants. Credit: WikiCommons CC0 1.0
Consider the example of the catalytic converter in your car. Inside a catalytic converter are billions of molecules of nitric oxide bouncing around. As these molecules bounce around, sometimes they will spatially align in just the right way so that the electrostatic interaction between neighboring oxygen and nitrogen molecules is strong enough to break the molecular bonds of nitric oxide and create molecules of nitrogen and oxygen. Without a catalyst, this reaction would occur at a very slow rate because the movement of the molecules is essentially random and they are unlikely to spatially align in just the right way. The platinum screens attract molecules of nitric oxide, aligning them in such a way that the reaction is more likely to occur. The platinum makes the overall rate of reaction faster because it makes individual reactions more likely to occur. Arranging molecules in an optimal geometry minimizes the entropy of the system, which allows the reaction to occur faster. Many enzymes in the bodywork in a similar fashion; they align molecules to make certain reactions more likely to occur.
Other times, enzymes work by allowing the production of different intermediary products which require less energy to be converted into the final products. Enzymes form a covalent bond with reactants to change their structure, which lowers the energy required for the reaction to proceed. For example, trypsin is an enzyme found in the digestive system of vertebrate animals. Trypsin works as an enzyme because it speeds up the conversion of ingested proteins into their constituent amino acids. Trypsin does this by cleaving the peptide bonds of proteins, creating smaller peptide bonds that require less energy to break down into amino acids. Without trypsin, this process would take much longer because larger peptide bonds require more energy to break. Additionally, without trypsin, some proteins could not even be broken down.
Other metal-containing enzymes work by stabilizing the electric charges of negatively charged reactants. Metals tend to form positively charged cations, which attracts negatively charged reactants. Metals can also serve as electron donors and acceptors because they readily accept and let go of electrons. Metal-containing enzymes are useful in biological contexts because they tend to be unaffected by changes in bodily pH.
Things That Influence Enzyme Reactions
The effectiveness of enzymes can be influenced by external factors. One thing that can affect the efficacy of an enzyme is temperature. In general, higher temperatures allow for faster reactions because the molecules are moving around more. Sometimes though, high temperatures can affect the structure of enzymes, making them unable to do their job effectively. Raising temperature too high can cause enzymes to denature and permanently lose their functionality. In most mammals, internal temperatures higher than 104 °F can cause enzymes and other proteins to spontaneously denature.
Enzyme effectiveness can also be affected by changes in pH. The active sites in enzymes have acidic and basic properties that help them do their job. Changing pH levels can cause residue to build upon these active sites, making it harder for the enzyme to bind to the right substrates. Too much change in pH levels can cause enzymes to denature.
To summarize, enzymes are a special kind of biological catalyst. Catalysts are chemical entities that accelerate the rate of a chemical reaction but are not themselves consumed in a reaction. Catalysts work by lowering the activation energy required for a chemical reaction to take place.
Enzymes are a special kind of biological catalyst found in living things. Enzymes are required in order for living things to produce the large amounts of energy required for survival by using the smallest amount of resources. The mechanisms by which enzymes lower activation energies vary depending on the enzyme. Some enzymes align molecules in geometric arrangements that are favorable for reaction while others provide an alternate chemical pathway for a reaction to occur.








