
Biodiversity conservation is a vital task for humanity as IPBES (1, see section 3.2, pp. 196) recently stated once again. The principle of optimal biodiversity (2, 3) is an attempt to help in this by returning to a biological basis and trying to understand why nature needs diversity.
If diversity is an adaptation
We suppose that the inner diversity (i.e. the diversity of the components) of a biological system is an adaptive trait associated with its effectiveness, resilience, and viability. Biological systems with optimal diversity are the most effective and viable. Any shift away from optimal diversity decreases the effectiveness and viability of a biological system or even eventually destroys it.
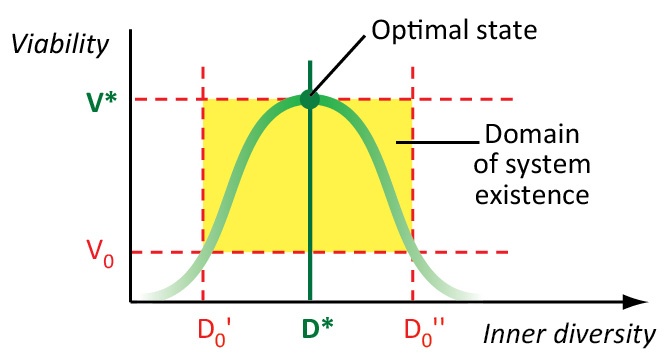
Figure 1. The optimal value of diversity (D*) corresponds to the maximum biosystem viability (V*). V0 – the critical value of viability; D0 – the critical value of diversity. Credit: Elena Bukvareva
Optimal biodiversity is established during an interaction of different hierarchical levels of life. Our theoretical models (2, 3, 4, 6) studied only a small fragment of life hierarchy, namely, populations and communities consisting of them, i.e. intrapopulation and species diversity. Further thinking comes from these models.
The opposite reaction of population and community levels – two sides of the same coin
The optimal values of intrapopulation and species diversity depend on the environment parameters (the amount of available resources and the degree of stability) in the opposite mode. Optimal intrapopulation diversity decreases in more stable conditions, while optimal species richness increases. Optimal species richness also increases in more “rich” conditions, and optimal intrapopulation diversity does not depend on the amount of resources. Intrapopulation and species diversity present two inseparable aspects of biological hierarchy, and the difference in their reaction indicates specific roles in the adaptation process.
Bottom-up optimization: components define diversity at the upper level
The optimal diversity values depend on the following population parameters:
– the width of the zone of ecological tolerance of individuals;
– the maximum population growth rate and mortality rate;
– the base resource costs of phenotypes and the cost of expansion of the tolerance zone.
Any improvement of each of these parameters that increases population viability leads to the same changes in optimal diversity, as stabilization of the environment. Thus, populations have different ways to compensate environmental instability: increasing a population growth rate, decreasing mortality, broadening individual tolerance zones, lowering the resource costs of phenotypes. These mechanisms can work within a population due to its adaptive capabilities, and within a community due to changes in the species composition, for example, shifting between K- and r-strategists and between specialists and generalists.
Improvement of any of the above parameters with the others remaining unchanged can be interpreted as an increase in the evolutionary level of organisms which, thus, may lead to a decrease in optimal intrapopulation diversity and an increase in optimal species diversity.
Expected patterns of optimal biodiversity
Intrapopulation diversity, other things being equal, can be considered a proxy of the width of an ecological niche. Thus, we can predict the optimal biodiversity structure of communities: 1) a large number of specialist species with narrow ecological niches in rich and stable conditions, 2) a small number of generalist species with wide ecological niches in scarce unstable conditions, 3) an intermediate optimal diversity structure in intermediate conditions (6). Undisturbed climax communities and their constituent populations seem to be the closest to optimal diversity. Some matching biodiversity patterns can be found both at the global and landscape scales (7).
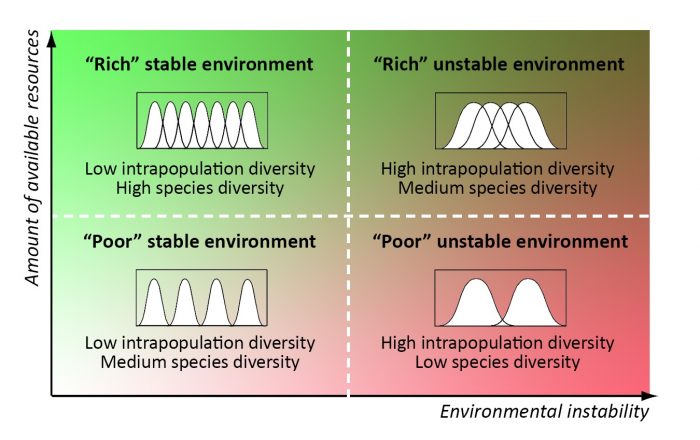
Figure 2. The expected values of optimal species and intrapopulation diversity in communities which are adapted to different environments. Credit: Elena Bukvareva
For nature management practice, this means that the criterion for conservation priorities should be the distance of an anthropogenic shift away from optimal diversity, but not high formal diversity indexes (e.g. species richness) because communities adapted to unstable or scarce conditions have relatively low species diversity which nevertheless ensures their maximum functioning.
The eternal pursuit of optimality
Can biosystems achieve the optimum diversity? It seems unlikely in our changeable universe. Any natural or anthropogenic changes in the environment or in biosystems themselves shift them from their optimal states to suboptimal ones. Thus, biosystems are in a permanent “pursuit” for optimal states during ecological, microevolutionary, and evolutionary processes (which may occur simultaneously).
An ecological example 1: the predominance of r-strategists in synanthropic biota.
The general direction of anthropogenic changes of the environment is destabilization (7) while the human impact on populations is expressed in a decrease in intrapopulation diversity. The latter reduces the possibility of populations to adapt to destabilization. As a result, adaptation starts to work at the community level and typical native species are replaced by other species shifting species composition from K-strategists to r-strategists and from specialists to generalists, which corresponds to a proliferation of synanthropic biota.
An ecological example 2: mechanisms of community assembly.
The prevalence of certain mechanisms in community assembly is determined by the ratio between “richness” and stability of the environment, or rather, by the ratio between the number of optimal niches that can exist under a given amount of resources and a given range of the environmental parameter (e.g. t °C) fluctuations (6):
1) competition and niche separation work predominantly if the number of optimal “resource” niches and the number of optimal “range“ niches are approximately equal (R/R*≈D/D*);
2) neutral mechanisms work predominantly in very “rich” environments where a great amount of resources allows the existence of a much larger species number than the range of the environmental parameter allows (R/R*>> D/D*);
3) “abiotic filters” operate mainly in a barren (harsh) environment where a small amount of resources allows the existence of a much smaller species number than the range of the environmental parameter allows (R/R*<< D/D*).
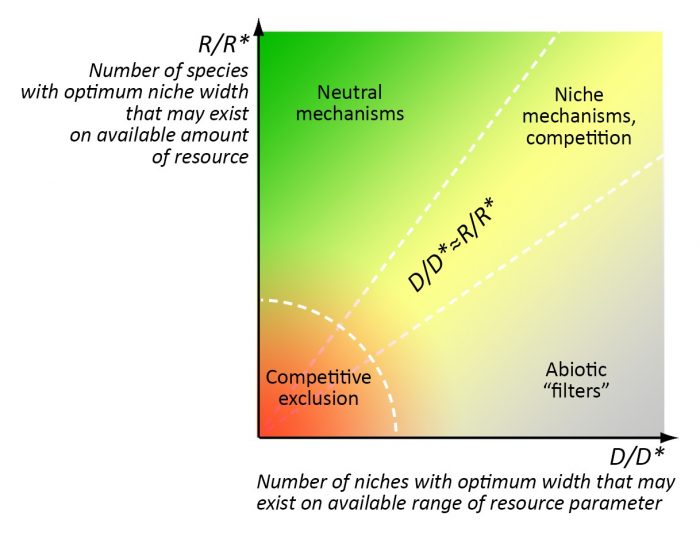
Figure 3. Zones of priority action of different mechanisms of community assembly. R is the total amount of available resources, R* is the amount of resources necessary for the population with optimal niche width, D is the total range of resource parameter, D* is the optimal width of ecological niche. Credit: Elena Bukvareva
A microevolutionary example: sympatric intraspecific forms as a mechanism of diversity optimization.
If the degree of environmental stability changes, a community must move between different optimal states: a large number of species with narrow niches in stable conditions and a small number of species with wide niches in unstable conditions. There are no problems if there are enough species in a regional pool and a community can easily include species with the necessary characteristics to achieve optimality. But what if a community is isolated or there are no suitable species in the regional pool? In this case, an optimization can occur due to a formation of sympatric intraspecific forms (ecotypes) when one species occupies multiple niches. This process can optimize both intraspecific diversity (the niche width) and community diversity (the species number). Sympatric intraspecific forms can work as a dynamic system, constantly adapting the local biodiversity to environmental changes until an isolation of forms leads to a formation of new species (3).
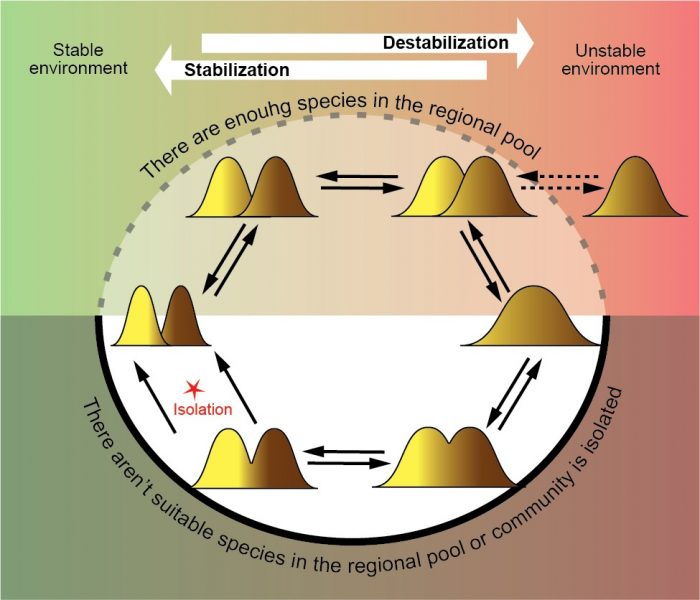
Figure 4. The dynamic cycle of optimization of community diversity when species exchange with a regional pool is sufficient (the upper part) and when a community is isolated. Credit: Elena Bukvareva
An evolutionary example: an overall increase in diversity despite ups and downs.
Biodiversity optimization may be an additional explanation of the processes within V. Zherihin’s concept of biocenotic regulation of evolution and V. Krasilov’s concept of coherent-incoherent evolution. Historical periods of environmental destabilization and destruction of communities (a biocenotic crisis) are characterized by an increase in intrapopulation diversity, expanding niches, extinction of specialized species, a decline in species diversity. An increase in intrapopulation diversity provides material for a future formation of intraspecific forms and speciation. Historical periods of environmental stabilization and development of “new generation” of communities are characterized by a decrease in intrapopulation diversity, narrowing niches, discretization of intraspecific forms, speciation, an increase in species number. An increase in the evolutionary level of organisms leads to a decrease in intrapopulation diversity (optimal niches became narrower) and an increase in optimal species diversity (see above) that may be an additional explanation of the general trend of an increase in species diversity during the evolution of life (3).
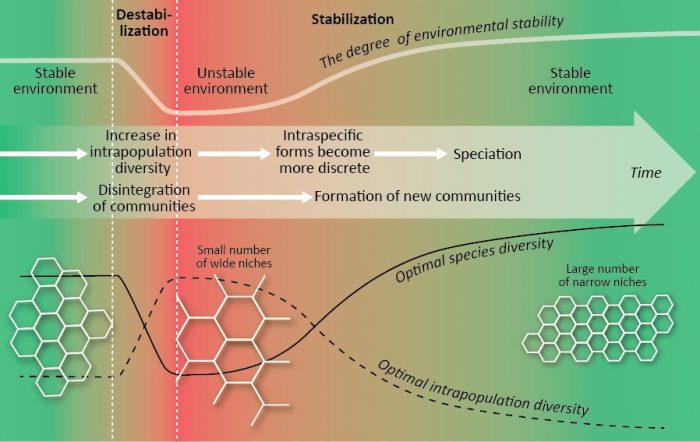
Figure 5. Changes in the optimal values of species and intrapopulation diversity during a biocenotic crisis and the subsequent development of new communities. Gray ” honeycomb” shows the optimal geometry of ecological niches. Credit: Elena Bukvareva
References:
- IPBES (2018): The IPBES regional assessment report on biodiversity and ecosystem services for Europe and Central Asia. Rounsevell, M., Fischer, M., Torre-Marin Rando, A. and Mader, A. (eds.). Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, Bonn, Germany. 892 pages.
https://ipbes.net/sites/default/files/2018_eca_full_report_book_v5_pages_0.pdf - Bukvareva, E., Aleshchenko, G., 2013. The Principle of Optimum Diversity of Biosystems. Association of The KMK- Scientific Publications, Moscow (in Russian). https://www.researchgate.net/publication/262344597_Princip_optimalnogo_raznoobrazia_biosistem
- Bukvareva E. 2014. The Summary of the Principle of Optimal Diversity of Biosystems. LAP Lambert Academic Publishing. 52 p.
https://sciencetrends.com/ - Aleshchenko, G., Bukvareva, E., 2010. Two-level hierarchical model of optimal biological diversity. Biol Bull Russ Acad Sci (2010) 37(1): 5–15. DOI: 10.1134/S1062359010010012
https://link.springer.com/article/10.1134/S1062359010010012 - Bukvareva E.N., Aleshchenko G.M. 2012. The Principle of Optimal Biodiversity and Ecosystem Functioning // International Journal of Ecosystem 2012, 2(4): 78-87. doi: 10.5923/j.ije.20120204.06
http://article.sapub.org/10.5923.j.ije.20120204.06.html - Bukvareva, E., Aleshchenko, G., 2013. Optimization, niche and neutral mechanisms in the formation of biodiversity. Am. J. Life Sci. 1 (4), 174–183. doi: 10.11648/j.ajls.20130104.16
http://www.sciencepublishinggroup.com/journal/paperinfo.aspx?journalid=118&doi=10.11648/j.ajls.20130104.16 - Bukvareva, E. (2018). The optimal biodiversity–A new dimension of landscape assessment. Ecological Indicators, 94(2), 6-11. doi:10.1016/j.ecolind.2017.04.041
https://www.sciencedirect.com/science/article/abs/pii/S1470160X17302170?via%3Dihub









