
Plant-parasitic nematodes infect the roots of plants, causing billions of dollars of crop loss worldwide. One such example is the soybean cyst nematode (SCN), which is the most damaging soybean pathogen in North America. Knowing the population density (number) of nematode eggs in the soil is crucial for the detection, monitoring, and effective management of the pest.
To determine the egg density present in a field, soil samples are collected from the field and nematode cysts (egg-filled dead female) are recovered from the soil using wet sieving and decanting or elutriation methods. Subsequently, the nematode cysts are ruptured, releasing the eggs which are captured on a sieve along with egg-sized debris. Traditionally, the debris is removed from egg suspensions using sucrose centrifugation, and then the eggs are stained and visually counted under a microscope to estimate the population density of eggs in the soil sample.
The problem is that most of the traditional egg suspension cleaning and counting steps are conducted manually and are time-intensive and laborious, thereby restricting the turnaround time and throughput. A limited number of soil samples can be extracted and analyzed in a given day. And while sucrose is inexpensive, sucrose centrifugation does not remove the debris completely, making counting the eggs difficult at times. Also, sucrose residue can remain on the surface of the eggs after centrifugation promoting the growth of contaminating fungi and bacteria in the samples when they are stored. A better egg sample cleaning process with automated egg counting was needed.
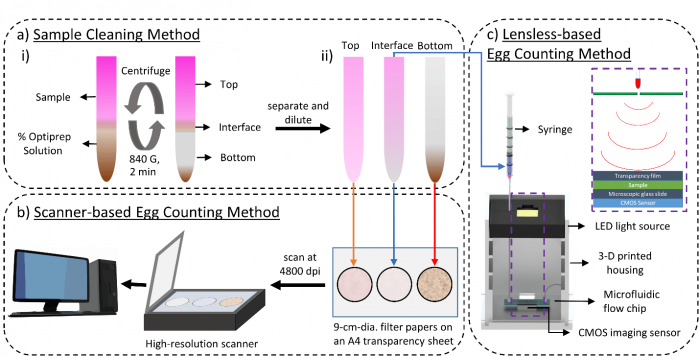
Figure reproduced with permission from PLOS from https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0223386
We developed an egg sample cleaning protocol using OptiPrepTM as the density gradient medium, instead of sucrose. For this, wet sieving and decanting were used to extract the eggs and debris from soil samples. The eggs were then stained to improve visualization among the debris. The egg suspension was mixed with OptiPrep, placed in 15 mL test tubes, and centrifuged at 840 G for 2 minutes. After centrifugation, most nematode eggs collected in the interface emulsion layer while the heavier debris particles deposited at the bottom of the tube. We determined the proper concentration of OptiPrep to obtain the best sample purification.
To automate the egg counting, the purified, stained egg samples were spread on filter papers and scanned with a high-resolution scanner. A deep-learning architecture using a convolutional autoencoder network searched through the scanned images to identify and count the nematode eggs in the sample. An alternative method of counting nematode eggs was developed by flowing the egg suspension through a microfluidic chip with lensless imaging. As the eggs and debris passed through the flow chip, a holographic video of passing objects was generated and saved. By reconstructing the holographic videos, the software was able to recognize the nematode eggs (even without staining) and keep a running count of the total number of eggs in the sample.
To test the performance of the new sample cleaning and imaging methods, soil samples were collected from two fields at Iowa State University research farms in Iowa that were infested with SCN. The egg suspensions extracted from soil samples were cleaned with sucrose centrifugation and with the new OptiPrep method. Then the egg preparations were stained and counted manually under a microscope as well as with the two new imaging methods we developed. The amount of debris in the samples and egg counts from the methods were compared.
The use of OptiPrep for sample cleaning significantly improved the egg recovery (> 80%) compared to the use of sucrose centrifugation (< 40%). And the results obtained from both automated egg counting methods were highly correlated with egg counts from direct microscope observations. Our automated egg counting methods required minimal human intervention only during sample preparation and loading. The software handled the remaining operations over a broad range of egg counts (10 to 300 eggs per mL). The cost of the hardware was intentionally kept low for wide adoption by nematology laboratories by using off-the-shelf components, adhesive tapes, and standard filter papers.









