
The molarity formula is one of the building blocks for understanding chemistry and chemical reactions. Being able to calculate the concentration of a solution is an important and basic piece of knowledge for a chemist. Calculating the molarity of a solution or using the equation for molarity to determine the quantity of a solute that must be weighed out in order to prepare the appropriate solution concentration is something that a chemist does multiple times a day.
Molarity is a value that expresses the concentration of a solute in a solution. The concentration of a solution depends on the amount of solute added to a volume of solvent. In chemistry, molarity is always expressed as the number of moles of the solute found in one liter of solvent. The formula for calculating molarity is as shown below:
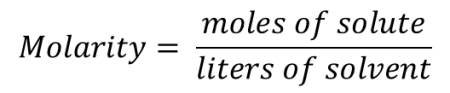
What is a Solution?
A solution is a mixture of two or more substances in which all of the components are completed dissolved and the mixture is uniformly composed. A fully uniform mixture is called a homogeneous mixture, or a solution.
What is a Solvent?
A solvent is very simply the substance into which the solute is dissolved. A solvent can be a solid or gas but is typically a liquid. The solvent is usually the component of the mixture that is present in the largest amount. In an aqueous solution, water is the solvent. When calculating molarity, the volume of solvent is measured in liters (L).
What is a Solute?
A solute is a chemical component of a mixture that is dissolved in the solvent. An example of a solute is sucrose when it has been dissolved into water to make sugar water. When calculating molarity, the amount of solute is measured in moles (mol).
What is a Mole?
A mole is a number in the way that a dozen is a number. One dozen is equal to twelve. A mole is equal to 6.02 x 1023, which is quite a bit larger than twelve. The number 6.02 x 1023 is called Avogadro’s number and is often written in scientific notation to consume less space. When fully written out, Avogadro’s number is 602 000 000 000 000 000 000 000, or 602 followed by twenty-one zeroes. It’s a big number but what exactly does it represent?
A mole of carbon atoms is 6.02 x 1023 atoms and this number of atoms weighs twelve grams because the relative atomic mass of carbon is twelve, as given by the periodic table. For other atoms, such as oxygen, a mole will weigh a different amount (in the case of oxygen, a mole of oxygen atoms weighs sixteen grams). A mole can be a number of molecules as well. Water is a molecule composed of two hydrogen atoms (relative atomic mass of one for each hydrogen atom) and one oxygen atom (relative atomic mass of sixteen), and a mole of water molecules weighs eighteen grams.
How is Molarity Calculated?
Molarity is calculated by dividing the amount of solute, which must be expressed in moles, by the amount of solvent, which must be expressed in liters. Below are some examples to help illustrate how molarity is calculated.
- Example 1: If you have 2.5 moles of sugar, and you dissolve the sugar into 0.5 liters of water, what is the molarity of the sugar water?
Molarity = moles of solute/liters of solvent
= 2.5 moles/0.5 liters
= 5 moles/liter = 5 mol/L = 5 M
Moles/liter can be expressed as mol/L or as simply M for molar.
As can be seen in the example above, when starting from moles, the calculation is quite straight-forward. However, moles aren’t something that can be directly measured, so often chemists begin with grams of a substance.
- Example 2: If you have 200 grams of salt (NaCl), and you dissolve the salt in 0.5 liters of water, what is the concentration of the salt water?
Molarity = moles of solute/liters of solvent, so how do we get moles of NaCl from grams? We use the molar mass of NaCl to convert the mass in grams to the number of moles. This conversion is possible because the molar mass of a compound like NaCl is calculated using the relative atomic mass of each atom from the periodic table and will tell us how much one mole of NaCl weighs.
NaCl molar mass = atomic mass of Na + atomic mass of Cl = 23.0 + 35.4 = 58.4 grams/mole
By dividing the mass of NaCl that we are dissolving into some water by the molar mass of NaCl, the grams units in both numbers cancel out, and we are left with moles only.
Grams of NaCl to moles of NaCl: 200 grams / 58.4 grams/mole = 3.42 moles
Molarity = moles/liter = 3.42 moles/0.5 liters = 6.84 mol/L
What is the Difference Between Molarity and Molality?
Where molarity is the number of moles of a solute found in one liter of solvent, molality is the number of moles of a solute found in one kilogram of solvent. The units of molality are mol/kg. Because the volume of a substance can be affected by temperature and pressure, using mass to convey quantity can provide a more accurate concentration.
- Example of a molality calculation: If 2 moles of sugar are dissolved in 0.5 liters of water, what is the molality of the solution?
Molality = moles of solute/kilograms of solvent
Because the density of water is 1 kg/L, 0.5 liters of water is equal to 0.5 kg of water. The density of solvents do not always equal 1 kg/L, though, so pay attention when calculating that unit conversion.
Molality = 2 moles of sugar/0.5 kg of water
= 4 mol/kg
Molality can be expressed as mol/kg, or a lower case m.
What is the Difference Between Molarity and Normality?
Normality is very similar to molarity, except that it is the number of mole equivalents found in one liter of solvent. Normality is used specifically for expressing the concentrations of acids or bases. The mole equivalents are the number of either protons (H+) or hydroxide ions (OH–) per molecule.
- Example of a normality calculation: What is the normality of 2 M of H2SO4?
The mole equivalents of H2SO4 is 2 since there are 2 protons in each molecule of H2SO4. By multiplying the number of mole equivalents by the molarity of the acid, the normality is calculated. So, the normality of 2 M of H2SO4 is 4 N.
Full knowledge of the properties of a solution includes the concentration of the solute, as well as the nature of the solute and solvent. The behavior of the solution will depend on these properties. With some simple math, many different properties of a solution can be calculated and used.









