Nitrate, chemical formula NO3, has a chemical charge of -1. Ion nitrates have a negative one formal charge. You may be wondering why this is the case. Why isn’t the full charge of N03 -9?
In order to understand this, let’s take a look at the number of atoms within a molecule of NO3 and understand how formal charges are calculated.
What Is A Formal Charge?
A formal charge, or chemical charge, is the charge that an atom possesses in a molecule, assuming that the electrons found within the chemical bonds are all equally shared amongst the atoms that make up the molecule. This means that relative electronegativity is not a factor.
In order to calculate a formal charge, electrons are assigned to individual atoms within the molecule based on two different rules: bonding electrons must be divided equally across the different bonded atoms, and non-bonding electrons are considered part of the atom they are located at.
The equation for determining the formal charge can be described as follows:
Formal Charge = eV – eN – eB/2
Given that:
- eV = The total number of valence electrons the atom possesses as if the atom were isolated from the rest of the molecule.
- eN = The total number of unbound valence electrons the atom has when positioned within the molecule.
- eB = The total number of electrons that are shared by the bonds that connect atoms to other atoms within the molecule.
Examples Of Formal Charge
Let’s look at an example of formal charge calculation:
Carbon dioxide, CO2, is a neutral molecule that possesses 16 electrons in its valence shell. Drawing the Lewis structure of the molecule reveals that it can be sketched out in three different ways. The carbon atom could potentially be joined to the oxygen atoms surrounding it with double bonds, or the carbon atom could be joined to one of the oxygen atoms with a double bond and to the other oxygen atom with a single bond. Finally, the carbon atom could be joined to both oxygen atoms with single bonds.
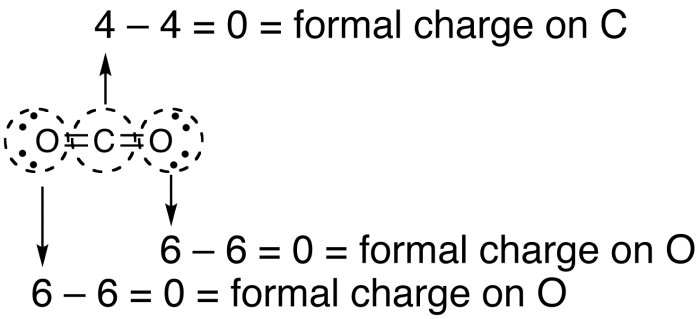
Example of calculations for formal charge of carbon dioxide. Photo: By Bkwan740 at English Wikipedia – Transferred from en.wikipedia to Commons by Ronhjones using CommonsHelper., Public Domain, https://commons.wikimedia.org/w/index.php?curid=16247065
Notice that in the case of carbon dioxide, every possible formation of the molecule has a formal charge of zero. In the first case carbon and oxygen both have a charge of zero, resulting in zero overall charges. In the second case, carbon has a positive 1 charge while the oxygen double bond has a charge of zero and oxygen single bond has a charge of -1 resulting in the net formal charge of zero. Carbon has a charge of +2 while the oxygens have a -1 charge each, again resulting in a formal charge of zero.
“The beauty of chemistry is that I can design my own molecular world.” — Ben L. Feringa
Let’s examine another example of a formal charge.
What is the formal charge of the compound tetrahydroborate, or BH4? BH4 is made out of boron and hydrogen, so let’s examine the bonds between the two elements. BH4 possesses no non-bond electrons, three valence electrons for boron, and four bonds around the boron atom. Translating this into a representation of the formal charge formula, the formula would be expressed as 3 – ( 0 + 4), or a total of -1 overall.
Now let’s examine the hydrogen atoms in the molecule. Like boron, hydrogen has zero non-bonding electrons, but it only has one valence electron and a single bond. This means that the formal charge of hydrogen within BH4 is 1 – (0 + 1), which means that the formal charge of hydrogen is zero.
Formal Charges And Lewis Structures
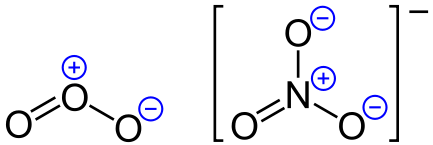
Examples of formal charges for ozone and a nitrate ion. Photo: By Jü – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=12017486
Lewis structures are also called electron structures, and they are diagrams which show possible bonds between the atoms that make up a molecule, as well as any electron pairs which are unbonded. The lines in a Lewis structure are drawn between atoms, and they are used to indicate the presence of chemical bonds. Single bonds are represented with single lines, while naturally double bonds are represented with double lines. Dots are occasionally drawn next to atoms to indicate the presence of unbonded electrons, and a pair of dots are representative of excess electrons.
Resonance structures are all of the possible different Lewis structures that a molecule might have. Calculating the formal charge for a molecule allows you to determine which resonance structure is more likely to be the molecule’s correct structure, and the Lewis structure which is considered the most correct will be the structure that has formal charges distributed evenly all throughout the molecule. When determining a formal charge as it relates to resonance structures, the sum of every one of the formal charges must equal the molecule’s total charge.
Summing Up Formal Charge:
A formal charge can be defined as the electrical charge of an atom within a molecule, and calculated by determining the total number of valence electrons minus half the total electrons located in a shared bond minus the number of electrons not in the molecule. You can use a formal charge to estimate the distribution of electrical charges in a molecule.
“The meeting of two personalities is like the contact of two chemical substances: if there is any reaction, both are transformed.” — Carl Jung
Facts About NO3 Nitrate
Nitrate is a salt comprised of nitric acid, and different alcohols, as well as esters, are sometimes referred to as nitric acid. Nitrate ions have a molecular mass of 63, and they are comprised of one nitrogen atom linked with three exactly the same oxygen atoms. Take note that nitrates are different from nitrites, which are salts made from nitrous acids, not nitric acid. Organic compounds that contain the nitro functional group are referred to as nitro compounds. Nitrate ions can be quite dangerous and toxic, and it affects humans through the process of nitrate toxicosis – a condition where iron atoms found in the blood’s hemoglobin are oxidized and become unable to carry oxygen.

A sample of sodium nitrate, or Chilean saltpeater. Photo: By Ondřej Mangl – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=2899599
Nitrates are rather commonly found in large deposits, especially deposits of nitratine. These nitratine deposits act as major fonts of sodium nitrate, also known as Chile Saltpeter. Nitrites are made by bacteria, and the nitrate compounds that this bacteria produces were historically used for gunpowder and produced by fermentation. Nitrates are also used in fertilizer because of their biodegradable properties and their high solubility. The primary nitrate fertilizers include calcium salts, potassium, ammonium, and sodium. Nitric acid is also produced during lightning strikes, owing to the interactions between water vapor and nitrogen dioxide.
What Is The Formal Charge Of NO3?
The Lewis representation of NO3 is aligned in a way where the nitrogen atom is in the center and orbited by three oxygen atoms. The nitrogen has a positive charge because it has 4 bonding electrons – 2 from the oxygen double bond and 1 from each of the N – O bonds. The inner core of the electrons is associated with just 6 electrons instead of the 7 that are needed for electrical neutrality, meaning it has a positive charge overall.
Since the nitrogen atom has only 6 electrons total, it, therefore, has a formal positive charge. The oxygens which have double-bonds have a shared or owned eight electrons, so they are considered neutral. Finally, the single bound oxygen atoms have nine electrons linked with them, and they have a negative charge overall. This means the nitrate ion has an overall charge of -1.
To put this another way, each oxygen atom has 2 electrons within their inner shell, and 6 electrons within the second shell of the atom. Oxygen has room for up to eight electrons in its second shell. The three oxygen atoms “want” to have 6 electrons. Meanwhile, nitrogen has 7 electrons total, five in the second shell and two electrons in its inner shell. Like oxygen, nitrogen has room for more electrons in a second shell, specifically, there is room for three more.
Since there are five electrons in nitrogen’s outer shell, nitrogen can be satisfied by giving these five electrons away and having only the two electrons in its inner shell. This frequently happens when sodium atoms interact with chlorine atoms. The atom of sodium donates a single electron from its outer shell to the chlorine, which forms table salt.
This means that nitrogen will donate its five outer shell electrons to the three oxygen atoms that surround it because the oxygen atoms have a greater pull on the outer electrons of nitrogen than nitrogen’s nucleus does. This means that the nitrogen atom will shed five electrons, but the three oxygen atoms want six electrons in total. So there will still be a desire for one last electron. In cases where nitrates are combined with other molecules, like when sodium combines with nitrate to form sodium nitrate or NaNo3, the single electron found in sodium’s outer shell will be transferred over to N03, which makes it a nitrate ion and ends up making it have a -1 charge.









