
What is the difference between a solvent and a solute? Both solvent and solute are parts of a solution. Solutions are mixtures of two or more substances, and the substance that dissolves into the solution is a solute. Meanwhile, the solute dissolves into a substance called the solvent. Solutes and solvents are mixed together to form many different products/solutions such as coffee, soap, ointment, and a variety of medicines.
Other important terms relating to solutions include “homogenous mixture” and solubility. Homogenous mixture describes a solution that has completely dissolved the solute, having the solute uniformly spread across the body of the solution. Solubility describes one substance’s ability to dissolve into another substance.
Defining Solute

Photo: By Chris 73 / Wikimedia Commons, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=11084
The solute is the substance that is dissolved into a solution. While most solutes are solid compounds, they can be a gas or liquid. Examples of solutes include sugar dissolved into water, salt dissolved in seawater, and oxygen dissolved into air. The attractive forces between the solvent and solute have to be strong enough in order to overcome the molecular forces that hold particles together, if the solute is going to dissolve into the solvent. As the solute dissolves into the solvent, it approaches a point called saturation. At the point of saturation, the solvent can’t dissolve any more of the solute – the solvent has reached peak concentration of the solutes.
As examples of solutes, consider how sugar dissolves into water. Sugar can be used to sweeten tea, and it does so by combining with the water and forming a solution. The sugar is that the solute and the tea the solvent. Another example is carbonated water, and here the water is a solvent and the CO2 the solute, and when the H2O and CO2 combine they form carbonic acid, which is responsible for giving carbonated water its slight acidity.
Notable characteristics of solutes: Solutes can be either gas, liquid, or solid, though they are often solids. Solutes typically have higher boiling points than solvents. The solubility of gaseous solutes is impacted by environmental pressure, in addition to temperature and volume. The solubility of something increases when the surface area of the solute’s particles increases.
Defining Solvent

The acetic acid in this bottle is a solvent. Photo: By W. Oelen – https://woelen.homescience.net/science/index.html, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=15691367
The solvent is what the solute dissolves into. Another way of defining the solvent is the substance that dissolves different compounds or substances in order to become a solution. Most of a solution is comprised of the solvent, with the solute only comprising a small portion of the solution. Solvents are usually liquids, though they can be other forms of matter. One of the most common solvents in the world is water, and it is capable of dissolving virtually any substance, giving it the designation universal solvent.
When considering which substances can dissolve into other substances, a heuristic you can use is the idea that “like dissolves like”. This refers to the fact that polar substances dissolve other polar substances more easily, while non-polar substances dissolve other non-polar substances more easily.
Polar solvents are the solvents that possess one or more electronegative atoms (like O, N, or H) and possess a high dielectric constant. Examples of polar solvents include carboxylic acids, ketones, alcohols, and amides. Polar solvents can be subdivided into two different categories, polar protic solvents, and polar aprotic solvents. Polar protic molecules include molecules such as methanol and water, and these molecules can create hydrogen bonds with solutes. In contrast, an example of a polar aprotic solvent is acetone, which cannot form hydrogen bonds with solutes. If a molecule like acetone is combined with a solute, dipole-dipole interactions occur instead.
Non-polar solvents are those that have bonds which contain atoms of similar electronegativity such as H and C. The structure means that non-polar compounds can easily be dissolved by non-polar molecules.
Notable characteristics of solvents include the following:
- Solvents typically have low boiling points and because of this, they evaporate easily in comparison to solutes.
- While solvents can be a gas or solid, they are often liquids.
- Common solvents frequently have carbon atoms within them and said carbon solvents are referred to as organic solvents.
- Solvents without the carbon element are called inorganic solvents.
- Examples of organic solvents include benzene, gasoline, acetone, alcohol, and xylene.
- Solvents play a role in regulating the temperature of the solution, either by increasing the speed that the reaction with the solute happens at or by absorbing the heat generated during the chemical reaction.
Reviewing the Differences Between Solvents And Solutes
To recap, solutes are substances that are dissolved by the solvent, melting into the solution. The solvent is what dissolves the solute, and for this reason, there is usually much more solvent than there is solute in a solution. Solutes can come in gas, liquid, or solid forms, and though solvents are primarily found in liquid states there are certain gaseous and solid solvents as well. The boiling point of a solute is to be higher than the boiling point of a solvent, and the properties of the solvent and solute are interdependent of each other.
A Look At Solubility
The solubility of an object or substance refers to the maximum amount of that substance that can dissolve into another substance. To put that another way, when a solvent is at equilibrium, the solubility of a substance is the maximum amount of solute it can absorb. When a solvent absorbs its maximum amount of solute, it makes a saturated solution.
Under the correct conditions, a supersaturated solution can be produced by dissolving additional solute beyond the point of equilibrium solubility. Once this point of super saturation is reached, adding more solute to the solution will not increase the solution’s concentration, and the solute that cannot be resolved will begin to precipitate out (see below). Be aware that although the concepts are sometimes confused, the rate of solution and solubility are not the same thing. The rate of solution describes how fast a solvent dissolves a solute, while solubility is the maximum amount of a substance that can be is dissolved. Solubility is also not equivalent to a substance’s ability to dissolve another substance when prompted by chemical reactions. HCl can dissolve zinc metal through a process of displacement with the result of the displacement reaction being the release of hydrogen gas and ions of zinc in the solution. So while zinc ions are considered soluble in acid the solubility of zinc is not a factor in the reaction.
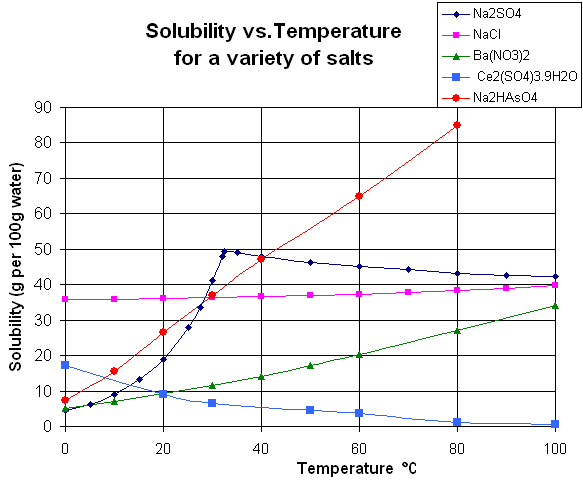
Photo: Public Domain, https://commons.wikimedia.org/w/index.php?curid=1608561
A substance is said to be insoluble when it dissolves poorly into a solvent, although even most insoluble solutes will still dissolve somewhat. There are only a few cases in which the solute going to dissolve at all. Generally, a solute is considered insoluble if less than 0.1 g of the solute dissolves for every 100 mL of solvent.
The types of chemical bonds in the solute and solvent affect how the solute will dissolve. As an example, new hydrogen bonds are created when ethanol and water molecules are combined together, even though ethanol sheets is molecular identity when dissolved into water (it stays as ethanol). Because of this, combining the water and ethanol creates less solution overall than would result from simply summing together the starting volumes of water and ethanol. When compounds like sodium chloride, ionic compounds, dissolve into water the compounded dissociates, breaking down into its constituent ions. These ions have water molecules surround them, becoming solvated. Dynamic equilibrium is involved in the solubility of a solution, which involves the opposing processes of dissolution and precipitation. The solution is considered to be at equilibrium when the two processes occur at the same rate.
The solubility of different solvents in compounds at various temperatures and other conditions are listed in solubility charts and tables. Solubility is defined in relation to a solute’s proportion to a solvent, according to the IUPAC. Concentrations can be given in units like molarity, molality, mole fractions, mole ratio, mass per volume, etc. The solubility of a substance can be influenced by a variety of different factors, such as the possible presence of other chemicals within the solution, the temperature of the solution, pressure, the phase of both the solvent and solute, polarity and solute particle size.
On Precipitation Reactions
Precipitation reactions are a type of chemical reaction where two soluble salts combine together in an aqueous solution and one of the resulting products is a precipitate (an insoluble salt). The precipitate can be separated out of the rest of the solution by filtration or centrifugation, or it can fall out of the solution over time, or even just stay in-place in the solution. The term for the remaining liquid after the formation of a precipitate is “supernate”. The likelihood that a chemical reaction will form a precipitate is influenced by the solubility of the substances involved, and the behavior of the solution can be predicted by consulting solubility rules or a solubility table. In general, iodides, bromides, and chlorides are soluble, and so are nitrates, acetates, and perchlorates. Also soluble are substances containing ammonium cations and alkali metal salts.









