
There are three different outcomes that can occur when solutes and solutions are combined, there can be a dilute solution, a saturated solution, or a precipitate. The solubility rules are general guidelines for the solubility of chemicals that will help you predict the likely outcome of mixing an ionic solid with a solvent.
What is solubility?
The chemical property of solubility refers to a specific substance’s ability to dissolve within a solvent. Solvents are measured in terms of a solvent-to-solute ratio at equilibrium, or in other words how much solute is present within a solvent at equilibrium. What results from a solute dissolving into a solvent is something known as a saturated solution.
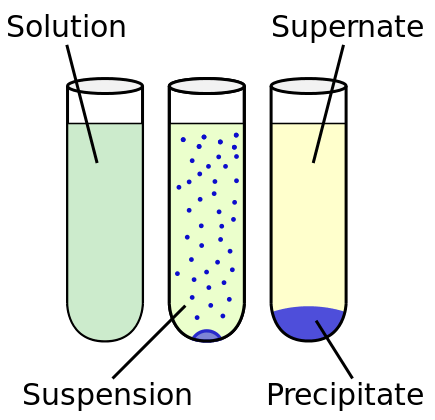
Photo: Vector by ZooFari; raster by ZabMilenko via WIkimedia Commons, Public Domain
Some substances, such as ethanol and water are soluble in every possible proportion when combined with the right solvent. To be soluble in all proportions is known as miscibility. If the equilibrium solubility of a solution is exceeded, which can happen in certain conditions, the solution is said to be supersaturated and is metastable in nature. Meta-stability refers to a state in a chemical/dynamic system that is stable, outside of the state of least energy, or ground state.
Certain conditions are needed for a solution to become supersaturated, such as the solution being at the correct temperature. As the chemical system becomes hotter, it tends to become more soluble, and as a result is capable of dissolving more solute into it then it could at lower temperatures. The various states of matter react differently to temperature changes.
Factors Influencing Solubility
While most solid substances have their solubility increase as temperature increases, this isn’t universally true. When liquid water is at extremely high temperatures, temperatures approaching critical temperature, ionic solutes become less soluble because of the changing properties of water at high temperatures. When solutes are in a gaseous form, they have hard to predict interactions with temperature increases. In general, though, gases become more soluble when combined with organic solvents at higher temperatures, while they become less soluble in water as temperature increases.
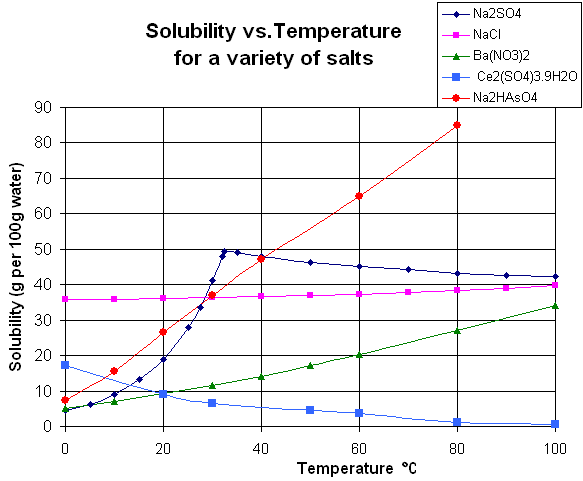
Graph depicting the relationship between temperature and solubility for various salts. Photo: Walkerma via Wikimedia Commons, Public Domain
Another factor influencing the solubility of a substance is pressure. The effect of pressure on solubility isn’t as great as temperature, and therefore it’s frequently treated as unimportant. However, it’s an important consideration when dealing with substances like calcium sulfate which sees its solubility decreased noticeably as pressure decreases.
Solvents are usually a solid or liquid, though not always. The solvent can be either a mixture or a pure substance. The system that the solvent dissolves into, the solute, can be solid, liquid, or gas. If a chemical is not soluble, it is referred to as insoluble. This term is also sometimes applied to compounds that are not easily soluble. There are very few compounds which aren’t soluble at all.
Dissolution
If solubility refers to substances ability to dissolve within the solute, then the actual process of the dissolving into a solvent is called dissolution. Dissolution is typically fairly simple when it comes to covalent chemicals. Covalent chemicals like ethanol create new hydrogen bonds when they dissolve in water. In contrast, the dissolution of ionic compounds can be more complex. Sodium chloride and other ionic compounds dissolve into separate ions when combined with water. These separate ions essentially just gain a coat of water molecules, becoming wrapped in them. Despite the fact that the sodium chloride ions don’t dissolve like ethanol dissolves, sodium chloride is still considered water-soluble, because when the solvent evaporates crystalline sodium chloride is left as a result.

Photo: By W. Oelen – https://woelen.homescience.net/science/index.html, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=15691367
When determining how likely a substance is to be soluble in another substance, the heuristic “like dissolves like” can be used. This refers to the general rule that solvents which have similar chemical structures as the solutes they are combined with dissolve said solutes the best. Take note that this is just a heuristic, and that there are exceptions to this rule.
In terms of measuring solubility, the solubility of a substance is often given as a concentration. The format is X amount of solute per kilograms of solvent, or X per 100mL of solvent.
Water Dissolves Certain Solids
Sucrose is the sugar that we use to sweeten drinks like tea and coffee. The carbon, hydrogen, and oxygen in sucrose are bonded together by inter-molecular forces, but these forces are fairly weak. When sugar is placed in the water, the bonds that hold the molecules together are easily broken up, dissolving the sugar into the water. That means that these C12H22O11 molecules end up being released into the solution.
Energy is required to break the bonds between the molecules within sucrose, and it also requires energy to break up the oxygen-hydrogen bonds in water. In order for the sucrose molecules to mesh into the solution, the hydrogen bonds in the water have to be disturbed. There are weak bonds that form between the solvent and the solute, and these can make up for the energy required to disturb the structure of the solvent and the solute. Sugar and water mesh so well together that a liter of water is capable of dissolving 1800 g of sucrose.
Salts, ionic solids, can contain negative and positive ions. These ions are linked together by the attraction between particles that have opposite charges. Ions become released into the solution as the solids dissolve into water. As a result, the ions associate themselves with molecules of polar solvents. In general, salts dissociate when dissolved in water, breaking up into their ions. Ionic compounds are capable of dissolving in water and the interaction of the ions and water molecules produces enough energy to compensate for the energy needed to break apart the ionic bonds of the salt. Furthermore, the energy needed to break apart the water molecules (so that the salt ions can be brought into the solution) must also be compensated for.
Equilibrium And Solubility
A chemical reaction is at equilibrium when there is no change in the concentrations of ions over time. When a system reaches the point of equilibrium, the solution is referred to as a saturated solution. It is called a saturated solution because the solution has the maximum amount of ions that can coexist with the solute in equilibrium. The amount of solute that has to be added to a given amount of solution in order for the solution to become saturated and hit equilibrium is referred to as the solubility.
The Solubility Rules
There are three different outcomes that can occur when solutes and solutions are combined. When the solubility of a solute and the amount of the solute are exactly equal, the result is a saturated solution. However, if there is less solute than the amount that can be dissolved (the solubility of the chemical) it is considered to be a dilute solution. Finally, a precipitate forms when excess solute is crystallized. This occurs when there is more solute than can be resolved, which results in the remaining solute separating out from the rest of the solution.
Solubility rules are guidelines for the solubility of the most frequently found solids.
Rule 1: The various salts that are comprised of ions of group I elements such as lithium, potassium, sodium, cesium, and rubidium are generally soluble with a few exceptions. Also soluble are salts that contain ammonium ions.
Rule 2: Salts which have nitrate ions within them are soluble in general.
Rule 3: Salts that contain anions of chlorine, bromine, or iodine are soluble in general. Exceptions to this rule include halide salts, like those made out of Ag+, Pb2+, and (Hg2)2+.
Rule 4: Silver salts are insoluble in general. Notable exceptions to this rule include Ag(C2H3O2) and AgNO3 .
Rule 5: Sulfate salts are soluble, with a few exceptions such as BaSo4, PbSO4, CaSO4, SrSo4, and Ag2SO4.
Rule 6: Hydroxide salts tend to be only somewhat soluble, displaying different levels of solubility depending on the group of elements that comprise them. Group 1 hydroxide salts are soluble, while group 2 hydroxide salts are only a little soluble. Transition metal hydroxide salts, as well as Al3+ hydroxide salt, are insoluble.
Rule 7: Sulfides made out of transition metals are insoluble, including Ag2S, ZnS, CdS and FeS. Lead sulfides, arsenic, antimony, and bismuth, also have insoluble natures.
Rule 8: The Carbonates are usually insoluble, including the group 2 carbonates like BaCO3, SrCo3, and CaCo3. PbCO3 and FeCO3 are also insoluble.
Rule 9: Chromates, anions comprise of both oxygen and chromium such as PbCrO4 and BaCrO4, are typically insoluble.
Rule 10: Phosphates, like Ag3PO4 and Ca3(PO4)2, are usually insoluble.
Rule 11: Flourides are usually insoluble, as seen by substances such as PbF2, MgF2, and BaF2.









