
The Lewis Dot Structure for carbon dioxide can be represented like this:
o=C=o
But what exactly does this mean? What is a Lewis Dot Structure, and what do the symbols in carbon dioxide’s structure represent? Let’s go over the Lewis structure and find out how to interpret this representation of carbon dioxide.
How To Read A Lewis Dot Structure
The Lewis dot structure is drawn with letters that represent the atoms of the element, and then a number of dots or dashes surrounding these letters. Dots can be used to represent the shared electrons within the bonds of the atoms, but dashes can be used to represent covalent bonds as well. Lone pairs of electrons are shown surrounding atoms with dots, and double bonds are represented with double lines instead of the single lines that typically join atoms together in the molecule.
“We are like an atomic structure. We’ve got a causal body that’s linked together.” — Frederick Lenz
Creating A Lewis Dot Structure

Photo: By Jynto (talk) – Own work This image was created with Discovery Studio Visualizer., CC0, https://commons.wikimedia.org/w/index.php?curid=21004140

Photo: Ben Mills via Wikimedia Commons, Public Domain
The Lewis Dot Structure is a graphical representation of how electrons are distributed around the atoms which comprise a molecule. The reason for drawing/creating a Lewis dot structure is that it helps one predict the kinds of bonds, as well as a number of bonds, that can be formed around an atom. Lewis structures can be utilized to make predictions about the layout of a molecule. Though a Lewis structure may seem intimidating at first glance, it is actually fairly easy to understand a Lewis structure and to draw one yourself, if you break down the creation of the Lewis structure into simple steps.
The first step to drawing a Lewis structure is to determine how many valence electrons the molecule has in total. This involves counting up all the valence electrons in the molecule. Valence electrons are the electron’s the atom possesses in its valence shell or the outermost shell of the atom. Atoms have different shells or layers, and each of these layers has its own electrons. However, in chemistry, typically only the electrons within the valence shell can participate in the formation of a chemical bond. Since the electrons in the valance shell are the electrons that will form bonds with other atoms to create molecules, knowing the number of valence electrons is important to draw a Lewis structure diagram.
In the second part of drawing a Lewis dot diagram, one needs to determine the number of electrons that are required to make the atom satisfied or “happy”. In other words, the number of atoms required to fill the outer electron shell of the atom must be found. When the outer electron shell of the atom is at capacity, it is considered “happy” and doesn’t want any more electrons in the outer shell. A good heuristic one can follow to know how many electrons are needed by an atom of an element is the “octet rule”. The octet rule refers to the fact that elements up to those found in period four on the period table require eight electrons in their valence shell to be satisfied.
“Matter, though divisible in an extreme degree, is nevertheless not infinitely divisible. That is, there must be some point beyond which we cannot go in the division of matter… I have chosen the word ‘atom’ to signify these ultimate particles.” — John Dalton
Step 3 of creating a Lewis structure is determining how many bonds are possessed by the molecule in total. Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair. In step 2 the number of electrons needed to create a bond was determined, while in step 1 the number of electrons present in the valence shell was determined. In order to determine the number of bonds within the molecule, the total number of valence electrons should be subtracted from the number of electrons needed to fill the outer shell of the atom. Doing this will give you the required number of atoms needed to complete the octets. Since every bond requires two electrons, the number of bonds is calculated by dividing the number of electrons in half.
Step 4 of creating a Lewis dot structure is choosing a central atom for the other atoms to branch off from in the diagram. The central atom of a molecule is typically the atom with the highest electron valence of the atom with the lowest level of electronegativity. Determining the electronegativity of an atom can be done by consulting a table listing specific values or using the periodic table electronegativity trend to identify the value. The electronegativity trend refers to the fact that in general electronegativity decreases as one moves down the periodic table, following a group downwards, while it tends to increase as one reads across the table from left to right. Halogen atoms, as well as hydrogen atoms, usually appear on the outside of the molecule, and for this reason, they aren’t typically selected as the central atom.
After selecting a central atom, the next step is to draw a skeletal structure. You need to draw the central atom and then connect the other atoms to the central atom by drawing straight lines connecting the atoms together. The central atom of a molecule can join with up to four other atoms.
After drawing the atoms and the connections between them, the electrons should be placed around the atoms, drawn on the outside. The octets should be completed on the outside of the atoms. Since the outside of the atoms needs complete octets, if it is discovered that there aren’t enough electrons then this means that the skeletal structure that was drawn in step 5 has an improper alignment. Some trial and error may be necessary at first in order to find the correct structure, although with practice this will become easier.
Finally, the other electrons that haven’t been used should be positioned around the central atom. Once the octet of the central atom is completed, any bonds left over should be used to create double bonds, which are represented by two straight parallel lines, instead of just one line. You can tell if the number of atoms was incorrectly counted in Step 1 if the central atom possesses more than eight electrons and the atom isn’t one of the noted exceptions to the octet rule.
How Are Lewis Structures Different From Real Molecules?

Photo: By Jynto (talk) – Own work This image was created with Discovery Studio Visualizer., CC0, https://commons.wikimedia.org/w/index.php?curid=21004140
Lewis structures are helpful models that make understanding the structure of atoms easier. However, like all models some amount of simplification/abstraction is necessary. Therefore there are some differences between real molecules and Lewis structures that should be noted.
One of the ways that Lewis structures and real molecules can be different is that atoms can create molecules that are not stable, even though in Lewis structures it is assumed that the atoms will seek to fill or have to fill their valence shell. The number of valence electrons can occasionally exceed eight, this is likely to happen when the atomic number of an element is especially high. Because the elements of higher atomic numbers occasionally have valence electrons which exceed eight, Lewis structures aren’t as helpful for creating models of transition metals as they are creating models of the light elements. The transition metals such as actinides and lanthanides are examples of transition metals that may have more than eight valence electrons. For these reasons, it is important to be aware that while Lewis structures can help you make sense of how atoms behave in molecules, it should not be assumed that the Lewis structure is a perfect representation of molecules.’
Reading The Periodic Table To Determine Number of Valence Electrons
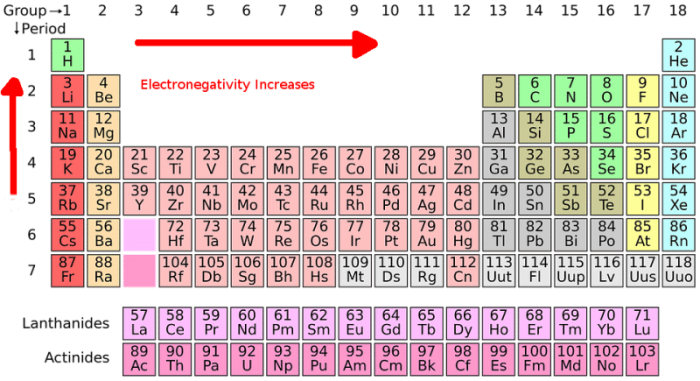
Photo: Geralt via Pixabay, CC0
You can examine the periodic table of elements to determine the number of valence electrons for a specific element. The elements in the periodic table are arranged in certain patterns, and noting the arrangement of the table can help you figure out which elements have how many valence electrons. The periodic table orders elements by chemical reactivity, or by numbers of valence electrons since valence electrons determine the chemical activity of the element.
“Wonder is the heaviest element on the periodic table. Even a tiny fleck of it stops time.” — Diane Ackerman
Columns or groups on the periodic table all contain elements that have equivalent numbers of valence electrons. For instance, take a look at the first group or column in the periodic table. This group contains hydrogen, cesium, sodium, and potassium among other elements. Every element in this first group has one valence electron. Moving over a column, the elements found within the group 2 of the periodic table, such as magnesium, beryllium, and calcium, all possess two valence electrons.
Note that the transition metals within the middle part of the periodic table are skipped in the grouping of elements by valence electrons, because of how the electron configuration in these elements works. With the exception of the transition metals, the trend across the periodic table holds true until the eighth and final column on the table is reached. The most stable elements are listed in this group or column, each possessing eight electrons in their valence shell already. These elements are referred to as the noble gases, and they include elements like argon, neon, and krypton.









