
Energy researchers have long been looking to the natural world for ideas about how to more efficiently harness energy and how to do in ways that emit as little pollution as possible. This is especially true regarding projects designed to create artificial photosynthesis, which is as the name implies are methods of drawing energy from the sun through artificial plant material. Recently, a team of researchers from the University of Cambridge succeeded in creating a similar, though not completely comparable, method of harnessing energy from the sun, which they have dubbed “semi-artificial” photosynthesis.
Semi-Artificial Photosynthesis
The researchers from Cambridge combined both synthetic and organic plant parts to create a system capable of splitting oxygen and hydrogen apart using modified plant photosynthetic systems. The researchers are hoping that their research can be applied to solar energy systems to boost their performance.
Katarzyna Sokół, the lead author on the paper and PhD student at Cambridge, explains how semi-artificial photosynthesis could possibly bridge the gap between synthetic materials and biology to create new forms of energy storage and sustainable energy conversion. Sokół explains:
This new field combines beneficial components of artificial systems such as electrodes, nanomaterials, synthetic dyes, and polymers with nature’s biocatalysts, such as enzymes, for the synthesis of solar fuels, such as hydrogen.

An example of a photo-electric cell. The cell has been given catalysts which are activated with sunlight, producing oxygen and hydrogen. Photo: MisterRichValentine via Wikimedia Commons, CC-BY-SA 3.0
The research team had to overcome a difficult problem with most artificial photosynthetic systems. Most artificial photosynthetic devices use synthetic catalysts to divide oxygen and hydrogen from one another, and these synthetic catalysts can be extremely expensive as well as toxic. The Cambridge researchers discovered how to implement organic enzymes as catalysts, overcoming this particular hurdle.
The enzyme in question is known as hydrogenase, an enzyme found in algae that can effectively distill protons into hydrogen. Over the course of its evolutionary history, algae had deactivated this ability since it wasn’t necessary for its survival, yet the researchers were able to reactivate the enzyme and achieve the reaction they sought.
The semi-artificial device uses synthetic components that are easy to tweak and customize by altering the organic catalysts found within plant cells, which makes an efficient and customizable system of semi-artificial photosynthesis able to convert solar energy and store it as a fuel. What kind of fuel can it be stored as? One of the ways that the energy can be stored is as hydrogen fuel.
Creating A Synthetic-Biological Device
The approach used by the Cambridge researchers was to use an electrochemical cell that functions very similarly to a battery. The electrochemical cells are based around the biochemical process known as photosystem II, which collects light. The electrochemical cells provide hydrogenase with the voltage they need to distill hydrogen. The hydrogenase reduces the hydrogen concentration in water, so it ends up becoming dissociated from the oxygen and filtering out as a gas.
One of the key differences in natural photosynthesis versus the hydrogenase/electrochemical cell reaction is that the semi-artificial method utilizes a wider range of the solar spectrum, which means it collects more energy. The process also manages to bypass a few different metabolic tasks, which isn’t usually doable using either biological materials or synthetic materials by themselves.
As for the hydrogen distilled by the system, it could be used to power hydrogen fuel vehicles and other systems. One of the advantages to using hydrogen as a fuel source is that unlike fossil fuels, which are becoming more scarce, hydrogen is extremely abundant, it just has to be extracted and put into a usable form. The primary limitation of hydrogen fuel is that the processes used to create it are themselves energy intensive and currently a large amount of fossil fuels are used to create usable hydrogen. This fact highlights why the research done at Cambridge is so important, it could provide scalable and affordable sources of hydrogen.

Photo: tuhinkhamaru740 via Pixabay, CC0
Beyond being used as a potential fuel source, this reaction could help remove carbon dioxide from the atmosphere, fighting the greenhouse effect and mitigating global climate change. Recently, researchers from the University of Central Florida discovered a way to use blue wavelengths of light to convert carbon dioxide to formamides and formate, two reduced forms of carbon dioxide that could be used as energy. Its an artificial method of photosynthesis that could potentially create solar fuel and reduce carbon dioxide in the atmosphere at the same time.
Sokół explains that the system created by the researchers is only a proof of concept and isn’t yet sophisticated or robust stable enough to be used in large-scale solar systems or for industrial applications. Yet in the future, the team intends to refine the technology and research whether or not the currently fragile enzymes can be replaced with more stable cells.
Other Research
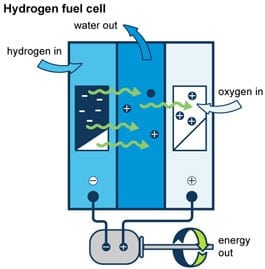
Photo: Adapted from the National Energy Education Project (public domain)
The research conducted at the University of Cambridge was not the only recent study relating to experiment with creating hydrogen fuel. Scientists from the University of Swansea recently devised a way to collect plastic waste and turn it into hydrogen fuel. The process functions through via cutting the plastic into many little pieces and then hitting the plastic bits with a photocatalyst. This photocatalyst uses the sunlight it absorbs and produces chemical energy.
When the plastic is put in an alkaline solution and stimulated with solar energy, it produces hydrogen gas. Another benefit of the process is that it can be used to degrade and recycle even greasy and oily plastic. An efficient way of collecting the hydrogen gas and turning into fuel will need to be developed, and the method of breaking down the plastic will have to be improved upon as well. The plastic fuel project will likely take a few more years of refinement before it is suitable for industrial applications, but the researchers are eager to continue their work and are currently investigating ways of scaling up the dimensions to of the project to more useful sizes.








