
Solar photoactive material like TiO2 has a wide range of applications. It can be used in photovoltaic (PV) cells, hydrogen generation, water treatment, and much more.
The demand for renewable energy resources is increasing day by day due to its environment-friendly nature, never-ending source, and cost-effectiveness. Various renewable fuels are available in nature and can be used for energy generation. However, hydrogen fuel is the most efficient in terms of energy content and pollution effect. Hydrogen is produced by different methods viz. water splitting and biomass conversion. A TiO2 catalyst is activated in the presence of UV irradiation and generates hydrogen via photocatalytic water splitting [1]. Water splitting using solar PV cell is also very popular.
Water is a priceless natural resource. Water is contaminated by various industrial effluents. This wastewater can be treated and recycled for further use. Water recycling reduces freshwater consumption as well as environmental pollution. The advanced oxidation process (using TiO2 and UV irradiation) is a very popular method for wastewater treatment. This UV/TiO2 process oxidizes the contaminants and produces less toxic final products [2].
For both the above-mentioned methods, TiO2 photocatalyst uses UV irradiation for its activity. This photocatalyst has a band gap of 3.2 eV. Therefore it will be activated in presence of UV light. We need to use artificial UV source since solar irradiation contains a very small fraction of UV. Utilization of visible light is necessary for the economic operation of these processes. Therefore, enormous research has been conducted to develop a visible light/solar-active TiO2 photocatalyst [3].
Photocatalytic activity of TiO2 depends on various factors such as structural, morphological, and optical properties. In a study by Singh and Dutta, a sol-gel process is used to synthesize TiO2. Proper controlling of the process parameters during the synthesis process enhanced the TiO2 quality since crystallinity and surface area play a vital role in the overall performance of the catalyst. Calcination temperature and pH are controlled to get optimum particle size and crystal size [3]. Figure 1. shows that the agglomeration rate is different at different temperatures. The synthesized TiO2 powder consists of fine particles and showed a lower rate of agglomeration that increases on further calcination at higher temperatures.
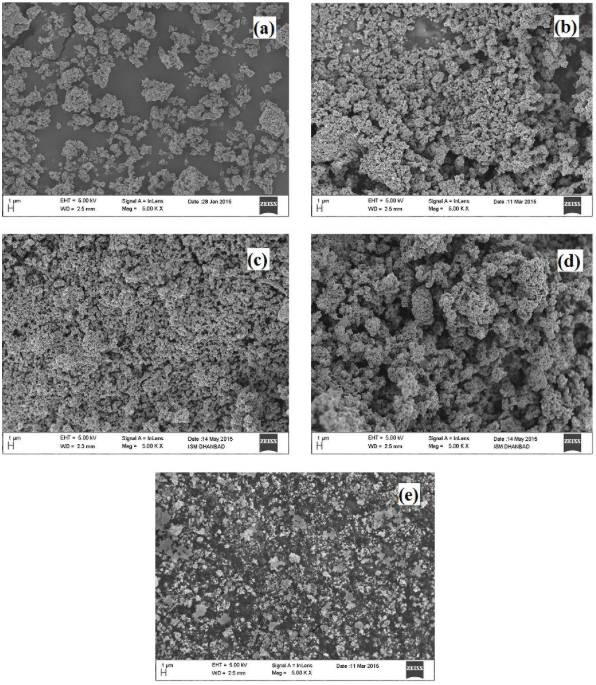
Fig. 1. FE-SEM images of prepared TiO2 nanoparticles (a) As-prepared (b) 450 °C (c) 600 °C (d) 900 °C (e) commercial TiO2 powder in terms of agglomeration rate [3]. Republished with permission from Elsevier from: https://doi.org/10.1016/j.apt.2017.11.005.
Characterization of the catalyst is important since it confirms the performance of the catalyst by determining the composition, crystal properties, band gap energy, particle size, surface area etc. Characterization is done using various methods such as XRD, FE-SEM, UV-vis spectroscopy, FTIR, Raman spectroscopy, and BET surface area [3].
Energy crisis and water pollution will be diminished by using materials like TiO2 and TiO2-based catalysts.
These findings are described in the article entitled Synthesis and characterization of solar photoactive TiO2 nanoparticles with enhanced structural and optical properties, recently published in the journal Advanced Powder Technology. This work was conducted by Rohini Singh and Suman Dutta from the Indian Institute of Technology (ISM), Dhanbad.
References
- Suman Dutta; A review on production, storage of hydrogen and its utilization as an energy resource; Journal of Industrial and Engineering Chemistry 20(4) 2014, 1148–1156
- S. Dutta, S.A. Parsons, C. Bhattacharjee, P. Jarvis, S. Datta, S. Bandyopadhyay; Kinetic study of adsorption and photo-decolorization of Reactive Red 198 on TiO2 surface; Chemical Engineering Journal 155 (2009) 674–679
- Rohini Singh, Suman Dutta; Synthesis and characterization of solar photoactive TiO2 nanoparticles with enhanced structural and optical properties; Advanced Powder Technology 29(2) 2018, 211-219
- Rohini Singh, Suman Dutta; A review on H2 production through photocatalytic reactions using TiO2/TiO2-assisted catalysts; Fuel 220(2018) 607-620









