
Hair loss is a common side effect of chemotherapy and radiotherapy for cancer treatment. It not only brings psychosocial stress to the patients but also compromises patients’ sense of personal identity. It ranks among the top concerns in patients undergoing cancer treatment and can potentially jeopardize the patients’ willingness for treatment.
Up to date, there is no effective treatment to prevent such hair loss. A team led by Sung-Jan Lin, deputy director of the Research Center for Developmental Biology and Regenerative Medicine and a professor in the Institute of Biomedical Engineering at National Taiwan University (NTU) in Taiwan, reported a new approach that targets stem cells in hair follicles. Their new approach reduces the damage of radiotherapy and chemotherapy to hair follicles in mouse models and may prevent hair loss in patients undergoing such treatments. Their report appeared in the September 22, 2017, online edition of the journal Cancer Research.
Hair follicles undergo life-long growth cycles, consisting of anagen (active growth), catagen (regression) and telogen (relative rest) phases. In human, at any given time, the majority of scalp hair follicles are in growing phase. In growing hair follicles, quiescent stem cells are maintained in the bulge region and hair bulbs at the hair follicle base contain rapidly dividing transit-amplifying cells (TAC).
These highly proliferative cells support the continued elongation of hair but also make human scalp hair follicles one of the most sensitive organs to genotoxic injury caused by radiotherapy and chemotherapy. Though growing hair follicles are sensitive to radio- and chemotherapy, they exhibit attempts to repair the damage before hair is broken. However, how this genotoxicity-induced follicular damage is repaired has long remained unknown. Lin’s team’s hypothesis is that enhancing the spontaneous reparative attempts can help to reduce the genotoxicity-induced follicular damage, thereby preventing hair loss.
Lin’s team traced different populations of cells that were genetically labeled with fluorescent proteins in transgenic mice to visualize their response to radiotherapy. They found that hair follicles are able to mobilize ectopic progenitor cells from distinct TAC compartments to repair the damage according to the severity of follicular injury caused by ionizing radiation.
They showed that, after a low dose of ionizing radiation injury, basal hair bulb K5+ progenitors were quickly activated to replenish the lost TACs in the hair matrix and quickly regenerated all concentric layers of hair follicles. Surprisingly, the bulge stem cells remained quiescent during the reparative process and did not contribute to the regenerative process. Physiologically, activation of bulge stem cells often requires complete hair follicle involution and lengthy resetting of the hair growth cycle. Due to the proximity of the K5+ progenitors to the lost hair matrix cells and without the need for hair cycle resetting, it provides a timely strategy for efficient regeneration.
Repairing Hair Follicles
Lin’s team also uncovered another group of cells, the outer root sheath cells, in growing hair follicles that acquire stem cell-like property to repair hair follicles. High-dose ionizing radiation depleted both hair matrix and hair bulb cells. In this condition, hair follicles generate new stem cells from outer root sheath cells to initiate a second attempt of repair. This showed that hair follicle cells located outside the conventional bulge stem cell niche retain the plasticity to be reverted to a stem cell-like state to repair damaged follicles in a more timely way.
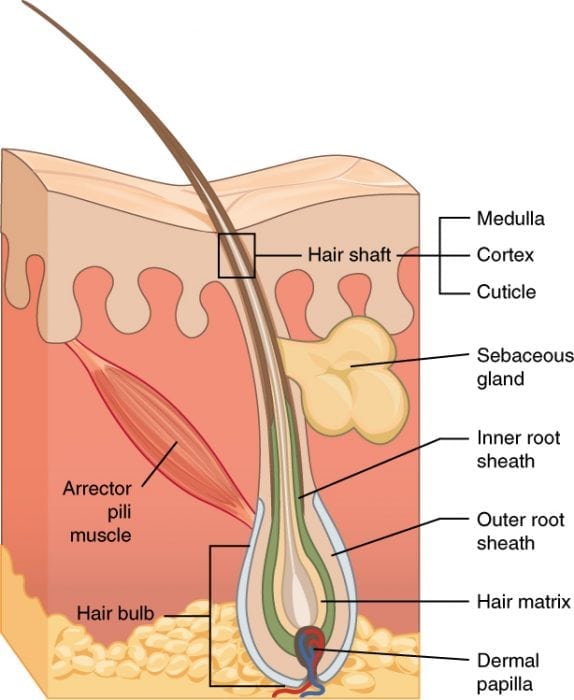
Hair follicle diagram. “506 Hair” by OpenStax College via Wikimedia Commons is licensed under the Creative Commons Attribution 3.0 Unported license.
The p53 protein is a cancer suppressive protein. P53 is activated by chemotherapy or radiation to kill cancer cells. Lin’s team found that p53 was activated in hair follicles following ionizing radiation. It not only induced hair follicle dystrophy and hair loss but also suppressed Wnt signaling and progenitor cell activation. Lin’s team then tested whether enhancing the mobilization of these ectopic stem cells can help to facilitate regeneration after chemotherapy and radiotherapy.
They found that enhancing Wnt signaling is able to bypass the suppressive effect of p53 and activates ectopic progenitor cells to promote hair follicle repair. They successfully prevented both radiotherapy and chemotherapy-induced hair loss by locally enhancing Wnt signaling in mouse skin. Their work represents a novel approach for preventing hair loss from chemo- and radiotherapy by targeting ectopic progenitor cells.
This study, Mobilizing transit-amplifying cell-derived ectopic progenitors prevents hair loss from chemotherapy or radiation therapy, was recently published in the journal Cancer Research.









