Research performed during the last 50 years has led to great progress in the therapy of cancer. Scientists were able to dramatically increase both the quality of life and lifespan of patients with multiple types of malignancies. This progress, however, in most cases, allowed oncologists to slow down the disease, but not to completely cure the patient of cancer.
The reason underlying this problem is the unique ability of tumors to evolve and change their properties over time. In simple terms, when a patient comes to the hospital for the next course of anti-cancer therapy, physicians have to deal with an entirely new tumor that behaves differently compared to the previous one. The tumor gradually acquires more and more aggressive phenotype and becomes harder and harder to treat. Why can cancer cells adapt so easily to new types of treatment and change their properties?
The reason for this is that animal cells over millions of years of evolution created multiple mechanisms to protect the body from injuries. In a normal situation, all these mechanisms increase our chances to survive. Unfortunately, cancer cells can easily use these pathways for their own benefits and, with its help, overcome treatment and evolve therapy resistance.
For the last 10 years, it was well documented that cells which died after an injury promote the growth of neighboring tissue to repair the damaged organ (1). An even more interesting phenomenon was observed in injured neurons. Neurons are the longest cells in the human body, reaching up to 1 meter in length. They have only one nucleus, and, therefore, if we cut neuron in two halves the part of neuron without a nucleus should inevitably die due to the lack of protein synthesis machinery. However, it is not always the case. It was shown that glial cells that surround neurons can use tiny membrane vesicles to send their own ribosomes and mRNAs to the damaged neuron and maintain it alive until it will be regenerated and reconnected with its nucleus (2).
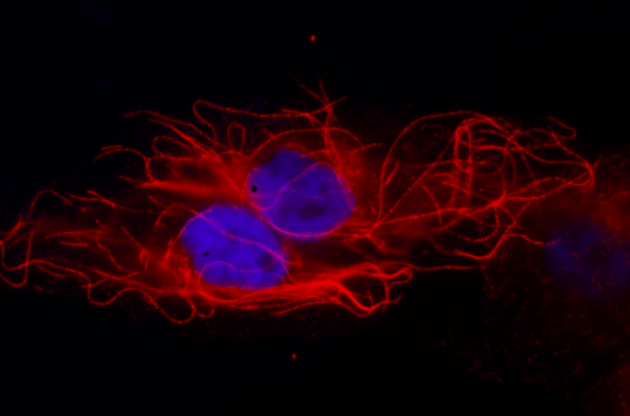
Credit: Marat S. Pavlyukov
In our two recently published projects (3, 4), we studied how cancer cells that die during therapy can affect the properties of the remaining tumor. With the help of a novel approach called Liquid chromatography–triple TOF mass-spectrometry, we analyzed clinical samples obtained from patients with ovarian and brain cancer and found that in both cases, tumor cells secrete a similar set of proteins after the chemo- and radiotherapy. These proteins were responsible for a process called RNA splicing.
Our cells contain only 25,000 protein-coding genes, while the amount of different proteins is estimated to be between 250,000 – 1,000,000. Therefore, on average, each gene can give rise to more than 10 different proteins. Most of these “additional” proteins appear due to the RNA splicing. In other words, during splicing, the cell cuts RNA molecules into pieces and then connects these pieces together in multiple different ways. Like changing the order of words in the sentences can completely change the meaning, RNA splicing can totally change the properties of the protein that will be synthesized from a spliced RNA molecule.
In our lab, we, for the first time, demonstrated that dying brain cancer cells can secrete “unusual” protein complexes responsible for RNA splicing (spliceosomes) inside tiny membrane vesicles. The vesicles can transfer spliceosomes to the neighboring surviving cancer cells where this “unusual” spliceosomes will change the way a cell splices its RNA molecules and will create new protein isoforms.
Further analysis revealed that spliceosomes from dying cells primarily affect genes involved in DNA repair and stress response. Changes in splicing of these genes lead to the appearance of more oncogenic proteins, which in turn promote therapy resistance, cell growth, migration, and more aggressive mesenchymal phenotype of brain cancer cells. Therefore, our finding raises an intriguing possibility that dying cells can send a signal to neighboring surviving tumor cells to prepare them for subsequent therapeutic insults.
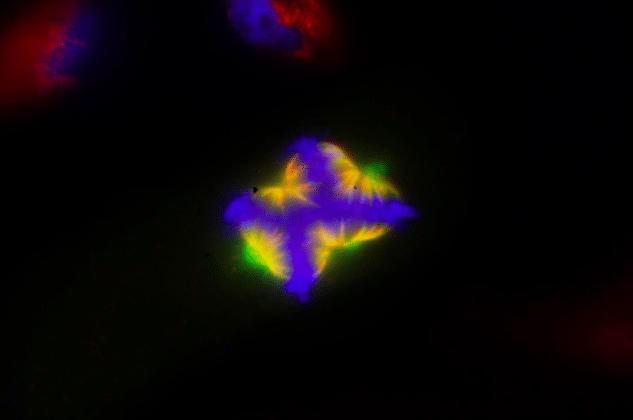
Credit: Marat S. Pavlyukov
Ongoing work in our laboratory is aimed to find a way to inhibit malignant splicing changes in cancer cells and therefore overcome therapy resistance and prevent the acquisition of a more aggressive phenotype of brain cancer cells.
These findings are described in the article entitled Apoptotic cell-derived extracellular vesicles promote malignancy of glioblastoma via intercellular transfer of splicing factors, recently published in the journal Cancer Cell journal. This work was conducted by a joint team of scientists from the USA (University of Alabama at Birmingham, Ohio State University), Russia (Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Federal Research Center of Physical-Chemical Medicine), China (Xi’an Jiaotong University, Huazhong University of Science and Technology) and Korea (Sungkyunkwan University, Chonnam National University).
References
- Pérez-Garijo, A. and Steller, H. (2015). Spreading the word: non-autonomous effects of apoptosis during development, regeneration and disease. Development. 142, 3253-3262.
- Court, F.A., Hendriks, W.T., MacGillavry, H.D., Alvarez, J., and van Minnen, J. (2008). Schwann cell to axon transfer of ribosomes: toward a novel understanding of the role of glia in the nervous system. J Neurosci. 28, 1024-11029.
- Shender, V.O., Pavlyukov, M.S., Ziganshin, R.H., Arapidi, G.P., Kovalchuk, S.I., Anikanov, N.A., Altukhov, I.A., Alexeev, D.G., Butenko, I.O., Shavarda, A.L., et al. (2014). Proteome-metabolome profiling of ovarian cancer ascites reveals novel components involved in intercellular communication. Mol Cell Proteomics. 13, 3558-3571.
- Pavlyukov, M.S., Yu, H., Bastola, S., Minata, M., Shender, V.O., Lee, Y., Zhang, S., Wang, J., Komarova, S., Wang, J., et al. (2018). Apoptotic Cell-Derived Extracellular Vesicles Promote Malignancy of Glioblastoma Via Intercellular Transfer of Splicing Factors. Cancer Cell. 34, 119-135.









