
The Lewis Dot Structure for O2 or dioxygen is as follows:
O = O
It’s a very simple structure, but how does one interpret this Lewis structure? How can one draw a Lewis structure and use it to understand how atoms bond together to make molecules? Let’s go over how Lewis structures are interpreted and drawn.
Facts About Oxygen (O2)
O2 is an allotrope of oxygen and is made out of two oxygen atoms bound together. Although the chemical formula for this allotrope is O2, it is frequently just referred to as oxygen. O2 or dioxygen’s particular formulation is one of the most common elemental compounds on the planet, constituting around 20.8% of the Earth’s atmosphere. Dioxygen (O2) is used in cellular respiration by many living organisms, used to create energy along with sugars.
How To Interpret A Lewis Structure
Lewis structures are diagrams that represent atoms and the bonds between them. The letters represent the atoms found within the molecule, with specific letters representing different elements. Meanwhile, dashes represent the bonds between the different atoms. Dots can also be found within the Lewis structure, either use to represent bonds (much like dashes) or used to signify lone pairs. Lone electron pairs are frequently represented with dots surrounding individual atoms. Meanwhile, double bonds are represented with double lines, naturally extending the idea that single lines represent a single bond between atoms.
“We may say that a basic substance is one which has a lone pair of electrons which may be used to complete the stable group of another atom, and that an acid is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms.” — Gilbert Newton Lewis
The octet rule is a rule which states that the electron configuration of noble gases can be easily achieved through the formation of electron-pair bonds between atoms. Many atoms have electron pairs in their octet which are not shared between different atoms, pairs found on their own. For this reason, these non-bonding electrons are referred to as lone pairs. While lone pairs aren’t involved in the formation of bonds between atoms, Lewis structures should always be drawn with lone pairs reflected.
How To Draw A Lewis Dot Structure

Photo: By Leyo – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=14816508
Because Lewis structures are just graphical representations of the bonding between various atoms, they can be used to help predict how atoms will bond together to create molecules. Lewis structures can assist one in understanding how electrons bond together, and how the layout of the molecule is affected by the number of electrons present within the electron’s valence shell. Drawing the Lewis structure yourself can make understanding and interpreting Lewis structures easier, and therefore breaking down the creation of Lewis structure into a few simple steps is advised.
The first part of creating a Lewis structure is analyzing the molecule as a whole and counting how many valence electrons the molecule possesses in total. All the valence electrons in the molecule must be accounted for. Valence electrons are the electrons found in the outermost shell of the atom, which is referred to as the valence shell. Atoms possess different layers of shells, and each of these layers has its own number of electrons. Yet the electrons found in the inner shells aren’t usually taken into account when analyzing the bonding of atoms because, usually, only the valence shell electrons can form bonds with other atoms. Because the valence shell electrons are the electrons that create molecules, it’s important to know how many valence electrons the molecule possesses in total in order to draw a Lewis structure.
“The beauty of a living thing is not the atoms that go into it, but the way those atoms are put together.” — Carl Sagan
The second phase of drawing the Lewis diagram is determining how many electrons and a given atom requires to be happy or satisfied. Atoms must have a certain amount of electrons in their outer shell to be satisfied, or not desiring any more electrons in the outer shell. While the outer shell of the electron isn’t necessarily at capacity when in this state, adding more electrons becomes increasingly difficult. A heuristic one can utilize to determine how many electrons are required by an element to be satisfied is the octet rule, which refers to the fact that many of the elements found in the periodic table, the main-group elements, tend to require eight electrons within their outermost shell to be satisfied.
Part three of creating a Lewis dot structure is calculating the number of bonds the molecule has overall. Covalent bonds are the bonds that form electron pairs, created when the electron of one atom joints with the electron of the other atom in the bond. When doing this, remember that you determined the number of electrons necessary to create a bond back in Step 2 of creating the Lewis structure. You should also know the number of electrons present in the valence shell of each individual atom since you calculated this in Step 1. It should be fairly easy to determine the overall number of bonds within the molecule, as all you have to do is subtract the number of atoms that the octets require to be completed from the total number of valence electrons. Be sure to divide the number of electrons in half because two electrons are required for every bond.
The fourth step in the creation of a Lewis dot structure is selecting a central atom. The central atom is the atom that the other atoms will branch out from. As mentioned above, the central atom in the Lewis structure is usually the atom that has the lowest electronegativity or highest electron valence. One can use the electronegativity trend on the periodic table to identify the electronegativity of a given atom. There are also tables that have specific values for electronegativity that one can consult. The electronegativity trend describes a trend found on the periodic table where electronegativity increases as one follows the table from left to right and decreases as one follows the table downwards. Halogen atoms and hydrogen atoms usually aren’t selected as the central atom because they typically appear on the outside of the molecule.
Once a central atom has been selected, the skeletal structure of the molecule can be drawn out. Start by drawing the central atom and then draw the atoms that surround it. Connect the surrounding atoms to the central atom with lines representing bonds. The central atom of a molecule is capable of joining with up to four other atoms. After the central atom has been drawn, along with its connections to other atoms, you can place electrons around the atoms. The unbonded electrons should be drawn on the outside of the atoms. Complete octets are required on the outside of the atoms, which means if you suddenly discover that you don’t have the correct amount of electrons to go around, the skeletal structure that was drawn before was improperly aligned.
It may be necessary to experiment at first, learning the intricacies of drawing the structure by trial and error, though this should become easier with practice. After you have drawn the central atom and its branching atoms, the electrons that haven’t been utilized should be drawn around the outside of the central atom. The completion of the octet means that any bonds which are leftover should be made double bonds, which you can represent by drawing two lines parallel to one another. If the atom isn’t one of the exceptions to the octet rule but it possesses more than eight electrons it is likely that an error was made in the counting of electrons during Step 1 of the process.
Differences Between Lewis Structures And Real Molecules
Creating a Lewis structure helps make intuiting the formation and structure of molecules easier. Yet it is important to know that models such as the Lewis structure require a degree of simplification, and therefore there are differences between Lewis structures and the structure of molecules in the real world. One of the ways that real molecules and Lewis structures are different is that atoms are capable of forming unstable molecules. Meanwhile, when Lewis structures are created the assumption is that the atoms will have filled, or are seeking to fill, their valence shells. The number of electrons in the valence shell of an atom is more likely to exceed eight when the atomic number of an element is higher.
“We are all just bi-products of atoms which are trying to understand itself and the limits of its capabilities.” — Dido Stargaze
Elements with higher atomic numbers are more likely to have valence electron numbers exceeding eight, and because of this Lewis structure aren’t usually made out of molecules of transition metals, since they frequently have more than eight electrons in their valence shells. Transition metals like lanthanides and actinides happen to be examples of elements with more than eight valence electrons. For these reasons, one should remember that although Lewis structures can be extremely helpful in understanding how molecules form, they do not perfectly represent how atoms interact to form molecules in the real world.
Determining The Number Of Valence Electrons With The Periodic Table
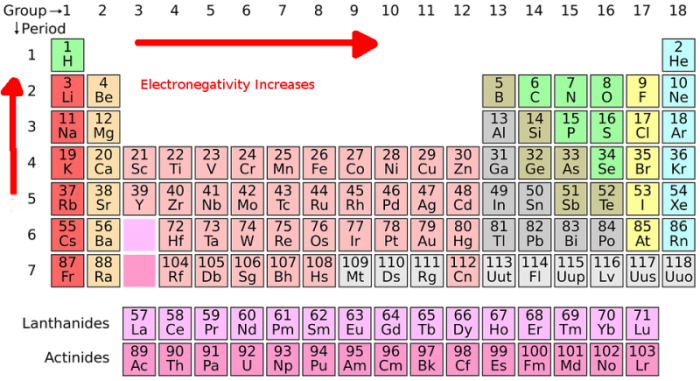
Photo: Geralt via Pixabay, CC0
As mentioned above, it’s possible to determine how many valence electrons are possessed by atoms of a specific element by consulting the periodic table of the elements. The elements found on the periodic table are arranged in specific patterns, columns, and rows. The groups (columns) on the periodic table are organized by how chemically reactive they are, or to put that another way by how many valence electrons atoms of the element possess.
Groups/columns on the periodic table of the elements all possess the same number of electrons in their valence shell. This can be confirmed by checking the number of valence electrons in the first group of the periodic table. Group one of the periodic table contains elements like sodium, potassium, hydrogen, and cesium. Every element group one of the periodic table has exactly one electron in its valence shell. Meanwhile, elements found within group two have two valence electrons, including elements like magnesium and beryllium.
This trend is referred to as the electronegativity trend, and it continues across the periodic table, with the exception of the transition metals in the middle of the table. These metals are skipped when grouping electrons by valence electron numbers. Outside of this exception, the trend across the periodic table holds true and can be used to determine the number of valence electrons an element possesses. Elements in group 8 of the periodic table already have electrons in their valence shell and they are referred to as the noble gases.










