Metalloids are elements found between the metals and nonmetals on the periodic table of the elements. They are also called semimetals. Metalloids have properties that are between the properties of nonmetals and metals. Most metalloids have:
- An appearance that is similar to metals
- They are less conductive than metal
- They are more brittle than metals
- Metalloids have nonmetallic chemical properties in general
Other properties of metalloids include: being good semiconductors, typically solid under ordinary conditions, can form alloys when combined with metals, typically act like nonmetals in chemical reactions.
“Each metal has a certain power… of setting the electronic fluid in motion.” — Alessandro Volta
Let’s take a close look at metalloids and discover what separates them from both metals and nonmetals.
Location Of Metalloids On The Periodic Table
70 metals, or metalloids, are found between nonmetals on the periodic table of the elements. Elements in this range have properties intermediate between nonmetals and metals. The exact elements considered to be metalloids are somewhat up for debate, with different classification systems considering different elements metalloids. However, the following elements are generally considered to be metalloids or semimetals:
Astatine, polonium, tellurium, antimony, arsenic, germanium, silicon, boron.
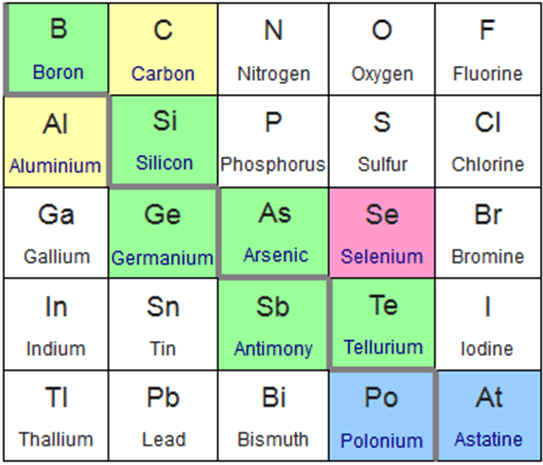
This slice of the periodic table highlight the elements classified as metalloids. The green elements are commonly considered metalloids, the blue elements irregularly considered metalloids, the pink considered metalloids less commonly than blue, and the yellow rarely recognized as metalloids. Photo: Sandbh via Wikimedia Commons, CC-BY 4.0
Tennessine or element 117 has not been analyzed in sufficient amounts to verify whether or not it is a metalloid, but scientists predict that it is one. Neighboring elements on the periodic table can have metalloid characteristics, and some scientists may categorize them to be metalloids. One example is carbon, which depending on the allotrope can be classified as either a nonmetal or metalloid. When carbon is in a diamond form, it behaves similar to a nonmetal, but graphite (another allotrope of carbon) acts as an electrical semiconductor and even has a metallic luster, so some consider it a metalloid. Other elements that have allotropes which can be metalloids or nonmetallic include oxygen and phosphorus. In environmental chemistry, selenium is often considered to be a metalloid. Other elements that may be considered metalloids when under the right conditions are: lead, gallium, bismuth, zinc, radon, iodine, sulfur, nitrogen, and hydrogen.
The ionization energies and electronegativities of semimetals/metalloids are in between nonmetals and metals, and as a result, metalloids have characteristics of both of these element categories. For example, although silicon has a characteristic metallic sheen, it is quite brittle and is an inefficient conductor. The element with which a metalloid reacts impacts the reactivity of the metalloid. As an example, when boron reacts with fluorine it reacts like metal, yet when boron reacts with sodium it reacts like a nonmetal. The densities, boiling points, and melting points of the metalloids vary widely. Because metalloids have an intermediate conductivity, they typically make good semiconductors.
Common Properties Of Metalloids
In general, metalloids share the following common properties:
- The electronegativities of metalloids are between those of nonmetals and metals.
- Ionization energies of metalloids are also between those of nonmetals and metals.
- Semimetals/metalloids have some characteristics of nonmetals and some characteristics of metals.
- The reactivity of metalloids depends on the properties of the elements they are interacting with.
- Metalloids tend to be good semiconductors.
- Metalloids may have a metallic luster, but they also have our tropes which can have a nonmetallic appearance.
- Metalloids are usually brittle, and they are also typically solid, only becoming non-solid under uncommon conditions.
- Metalloids typically behave as nonmetals in chemical reactions, and they can create alloys with metals.
Summing Up The Properties Of Metalloids

Displayed here are carbon’s graphite and diamond forms. Note the metallic sheen. Photo: By Commons: Robert Lavinsky – File:Diamond-and-graphite-with-scale.jpg, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=39385953
Summing up, let’s take a quick look at both the physical properties and chemical properties of metalloids. Physical properties are characteristics that can be documented or observed without altering the substance of the element, without changing the group of molecules into substances. Physical properties include things like the freezing point and density. Physical properties of metalloids are as follows:
- Metalloids have a solid state of matter.
- In general, metalloids have a metallic luster. Metalloids have low elasticity, they are very brittle.
- Middleweights are semi-conducted elements, and they allow leave the average transmission of heat.
Chemical properties are those which defined how a substance interacts/reacts with other substances or changes one substance to another substance. Chemical reactions are the only time that the chemical properties of an element can be quantified. Chemical reactions include things like rushing, burning, tarnishing, exploding, etc. The chemical properties of metalloids are as follows:
- Metalloids easily form gasses when they oxidize.
- Metalloids can be combined with metals to create alloys.
- Metalloids have different metallic and non-metallic allotropes.
- When metalloids melt some of them will contract.
- Metalloids can react with halogens to form compounds.
Facts About Metalloids
Oxygen is the most abundant element in the Earth’s crust overall, but silicon is the second most abundant element in the crust. Metalloids like polonium and arsenic are highly toxic. The electronics industry has great use for metalloids, with metalloids like silicon being used to make computer chips. Tellurium and antimony are used to give metal alloys desirable properties.
Structure Of Metalloids
When silicon crystallizes it forms a diamond shape in a crystal structure. Photo: By Ben Mills – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=2722584
Metalloids have a crystal structure that results from covalent bonding. Elemental silicon, antimony, arsenic, germanium, and tellurian have a lustrous shine, and therefore they look like metals. Germanium and silicon have a diamond structure when crystallized. The atoms within the crystal have covalent bonds anchoring them to four neighboring atoms at the corners of a tetrahedron. Massive three-dimensional molecules comprise single crystals of germanium and silicon. Arsenic has several different allotropes with the most stable allotrope having a layered structure composed of sheets of arsenic atoms. The arsenic atoms are bonded to three other atoms around them. Antimony and arsenic both have structures that are similar to the structure of graphite, arrayed in a lattice. Meanwhile, tellurium has crystals within it that contain infinite spiral chains of tellurium atoms.
Boron forms an icosahedron with boron atoms on every corner, and the crystalline structure is transparent. The most common arrangement of the atoms is one where they are extremely close together, with the boron-boron bonds being approximately 176 PM in length. There are other forms of the icosahedra as well, which have different arrangements of the boron atoms.
The silicon metalloid easily forms compounds with oxygen, creating bonds in the si-o-si format. These bonds are extremely important in the formation of minerals, somewhat analogous to carbon bonds which are of supreme importance in the formation of organic compounds in plants and animals.
Other Types Of Elements
Outside of metalloids, there are also metals and nonmetals. Metals are found at both the middle and left side of the periodic table of the elements. Group IA and Group IIA are metals, and another set of metals are the transition metals – groups IB to VIIIB on the periodic table. The primary metals are found to the right of the transition metals. The bottom two rows of elements are also metals.
“Base metals can be transmuted into gold by stars, and by intelligent beings who understand the processes that power stars, but by nothing else in the universe.” — David Deutsch
Metals are known for being dense and shiny and solid at room temperature, with the exception of mercury which is liquid at room temperature. They have high densities and high melting points. Metals are also known for having low electronegativity, a large atomic radius, and low ionization energy. These properties are the results of the electrons in the valence shell of the atoms, which can be easily removed. Another property of metals is the fact that they are malleable, they can be easily deformed and reformed without breaking. Finally, because valence electrons are able to move rather freely in metals, they are good conductors of both heat and electricity.
Nonmetals are found on the right side of the periodic table, and while both noble gases and halogens are nonmetals, the nonmetal element groups also include hydrogen, carbon, oxygen, nitrogen, phosphorus, sulfur and selenium. Nonmetals have high electronegativity in general, as well as high ionization energies. Nonmetals don’t conduct either electricity or heat very well. Solid nonmetals have no or very little luster and they are typically very brittle. Nonmetals can gain electrons easily compared to other types of elements.









