Our planet has a self-regulating ecosystem that stabilizes the temperature, climate, and other external environments. Water covers more than 70% of the land surface, and the water cycle plays an indispensable role in the earth’s environmental regulation. Coincidentally, the proportion of water in the human body is close to that of the earth’s surface. Undoubtedly, the circulation of water is vital to human health. Our kidneys work as superfluid percolator, filtrating approximately 50 gallons of initial urine every day. With the decline of the number of nephrons and the scar formation induced by various chronic diseases, the renal function will be irreversible and gradually decreased (Figure 1).
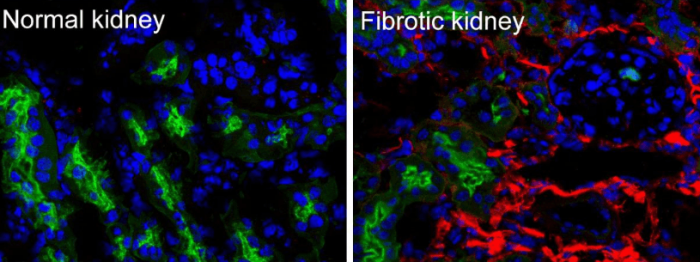
Figure 1-Collagen I deposits in a human fibrotic kidney. Collagen type I protein was labeled with red fluorescence, the proximal tubules were labeled with green fluorescence, and the cell nucleus was labeled with the blue fluorescence. (These unpublished photos are taken by Peng Wang and posted here with his permission)
TGF-β has been recognized as the most vicious mediator in the pathogenesis of renal fibrosis and plays an important role in promoting fibrosis in renal cells. TGF-β initiates its cellular actions by a signaling cascade effect; among this process, Smad3 phosphorylation has been recognized as a crucial step in TGF-β1/Smads signaling and even can be a potential target to slow down renal fibrosis.
Our exploration started by screening TGF-β signaling associated with long noncoding RNAs (lncRNAs) in tubular epithelial cells. During rounds of selection, we identified a kidney-enriched lncRNA, termed as lnc-TSI, that functioned as an endogenous inhibitor of TGF-β1/Smad3 pathway and regulated renal fibrogenesis. First of all, we found that lnc-TSI was up-regulated by Smad3 and, in turn, inhibited Smad3 phosphorylation in a negative feedback loop.
Next, we revealed that lnc-TSI bound the MH2 domain of Smad3 and blocked the interaction between TβRI and Smad3. This biological mechanism makes the role of lnc-TSI specific to Smad3. In animal experiments, delivering human lnc-TSI to mouse kidneys effectively alleviated renal fibrosis in two mouse models, suggesting that lnc-TSI acted as a guardian ameliorating renal fibrosis. Last but not least, in a cohort of patients with biopsy-confirmed IgA nephropathy (IgAN), lnc-TSI renal expression negatively correlated with the renal fibrosis index.
In the longitudinal study, 32 IgAN patients with low expression of renal lnc-TSI at initial biopsy had more pronounced increases in their renal fibrosis index and experienced stronger declines in renal function at repeat biopsy at a mean of 48 months to follow-up. These data suggest that lnc-TSI reduced renal fibrogenesis through negative regulation of the TGF-β/Smad pathway and may be used as a therapeutic target.
Chronic kidney disease (CKD) has become a “public health problem” all over the world. In 2010, 2.6 million uremic patients worldwide relied on dialysis or kidney transplantation for life support due to renal dysfunction, and 2.3~7.1 million patients worldwide died prematurely due to unavailable dialysis or kidney transplantation treatment. Therefore, any strategy that can prevent or delay the progression of chronic kidney disease to uremic has extremely important social and economic significance. The discovery of new targets to intervene in renal fibrosis is the first step toward the establishment of clinical prevention strategies.
The traditional consensus in biology is that proteins are the performers of all life processes. DNA and RNA are just the blueprints for protein synthesis. However, studies of the human genome have shown that the vast majority of DNA and RNA are not directly involved in protein synthesis. At present, increasing studies have confirmed that lncRNAs are involved in the regulation of various physiological and pathological processes, and their regulation form is more specific and flexible than protein. Our study shows that lncRNA can be applied as a “key node molecule” of the intracellular signal transduction pathway to regulate the pathophysiological process of diseases.
These findings are described in the article entitled Long noncoding RNA lnc-TSI inhibits renal fibrogenesis by negatively regulating the TGF-β/Smad3 pathway, recently published in the journal Science Translational Medicine.









