The development of luminescent materials has revolutionized human society, increased global productivity and improved the quality of life in dramatic fashion. Phosphorescent materials hold great promise in artificial lighting,[1] in photocatalysis to drive chemical transformations of compounds of industrial relevance,[2] and in sensing for the detection of a variety of analytes from nitroaromatic explosives to biological targets.[3]
Chemists have often found inspiration from the spontaneous and precise self-assembly exhibited by a giant and well-defined biological structures. In natural photosynthesis, for example, solar energy conversion is promoted through self-organized assemblies of functional chromophores. Similarly, secondary and tertiary structures of self-assembled proteins provide well-defined local environments to mediate biochemical reactions.[4] Much effort has been devoted in recent years to the preparation of large artificial nanostructures to mimic the precise assembly of multiple protein subunits into functional superstructures.
In an effort to develop novel photoactive nanomaterials, our group has recently focused on the self-assembly between metal ions and complementary luminescent scaffolds, aiming to create high concentrations of chromophoric units that, when assembled in well-defined geometrical arrangements, exhibit emergent photophysical properties.
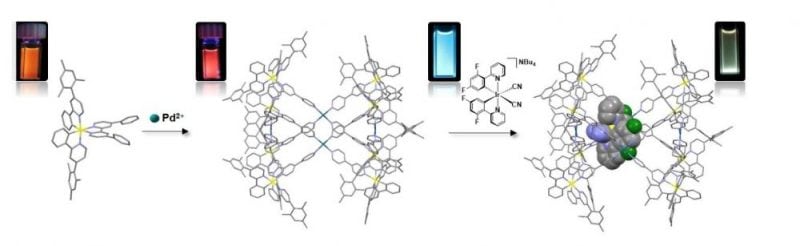
In this context, we have recently reported the first examples of homochiral red- and yellow-emitting supramolecular cages of the form of [Ir8Pd4]16+ based on the self-assembly between two families of enantiopure iridium(III) chromophores with Pd2+ ions (Figure 1).[5] Although it is well known that mononuclear iridium(III) complexes exhibit remarkable photophysical properties such as high photoluminescence quantum yields and good emission color tunability across the visible spectrum, this was one of the first examples that showed that the beneficial emission properties of Ir(III) chromophores can be transferred in self-assembled 3D-cage structures.
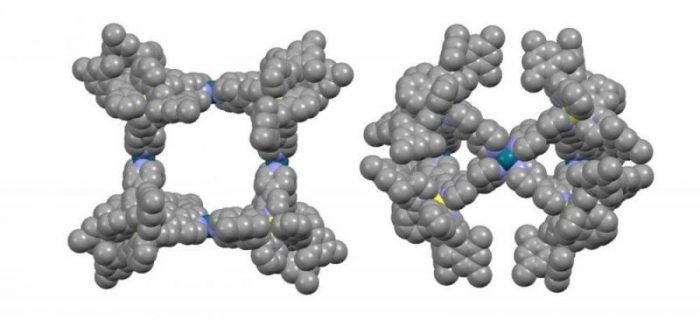
View of the structure of cage [Ir8Pd4]16+ with space-fill representation. Credit: Diego Rota Martir & Eli Zysman-Colman
In light of these results, we believe that our chiral photoactive cages [Ir8Pd4]16+ are promising candidates as chiral photoactive containers capable of absorbing photons and transferring light energy to or from encapsulated guest acceptors. These assemblies open up the possibility of promoting stereoselective photocatalytic transformations, examples of which at present are exceedingly rare. On the materials front, by encapsulating luminescent guests exhibiting complementary emission colors compared to the luminescent hosts, white-light emitting materials can be efficiently produced. This can be of great importance for designing novel solution-processed electroluminescent devices.
References
- C. Bizzarri, E. Spuling, D. M. Knoll, D. Volz, S. Bräse, Coord. Chem. Rev., ASAP, DOI: 10.1016/j.ccr.2017.09.011.
- C. K. Prier, D. A. Rankic, D. W. C. MacMillan, Chem. Rev. 2013, 113, 5322.
- D.-L. Ma, V. P.-Y. Ma, D. S.-H. Chan, K.-H. Leung, H.-Z. He, C.-H. Leung, Coord. Chem. Rev. 2012, 256, 3087.
- L. Yang, A. Liu, S. Cao, R. M. Putri, P. Jonkheijm, J. J. L. M. Cornelissen, Chem. Eur. J. 2016, 22, 15570.
- D. Rota Martir, D. Escudero, D. Jacquemin, D. B. Cordes, A. M. Z. Slawin, H. A. Fruchtl, S. L. Warriner, E. Zysman-Colman, Chem. Eur. J. 2017, 23, 14358.
- S. Horiuchi, H. Tanaka, E. Sakuda, Y. Arikawa, K. Umakoshi, Chem. Eur. J. 2016, 22, 17533.
This study, Homochiral Emissive Λ8- and Δ8-[Ir8Pd4]16+ Supramolecular Cages was recently published by Diego Rota Martir and Eli Zysman-Colmanin, among others in the journal Chemistry – A European Journal.








