
Microfluidic fuel cells (MFC) have been studied extensively since the last decade. Compare with conventional fuel cells, particularly Proton Exchange Membrane Fuel Cells (PEMFC) and Anion Exchange Membrane Fuel Cells (AEMFC), MFC could eliminate all the drawbacks associated with the membrane since it works by the co-laminar flow of the electrolyte which do not need a membrane to separate the anolyte and catholyte.
Fuel (methanol) oxidation and oxygen reduction reaction in the anode and cathode respectively required catalysts to facilitate the reaction rate. Most of the electrodes used in MFC is a carbon paper-based 2-D electrode. There were catalysts, which were nanoparticles for most of the case, supported on the carbon paper forming a 2-D plane for the redox reactions in electrodes. However, it only allows a thin layer of fuel to reach the electrode surface, which means a lot of fuels are flushed away and wasted. A porous anode which forces the whole anolyte stream to flow through itself could utilize the fuel much more efficiently. Moreover, due to the fact that the porous anode has a much larger surface area for growing the catalyst, the available active sites for methanol oxidation reaction is much more than the 2-D electrode and hence the specific power output is expected to increase.
MFC can be classified into two types, namely the co-flow and counter flow MFC. Since the two electrodes were in a face-to-face structure in co-flow MFC, the ionic resistance in the cell is smaller. In contrast, the anolyte and catholyte were well separated in the counterflow configuration, the fuel crossover can be well controlled with a compromised cell performance. Since the porous electrode itself is a resistance to the anolyte stream, the anolyte would flow to the catholyte stream rather than flow through the porous anode which cause severe fuel crossover in co-flow structure. To alleviate this problem, a novel orthogonal flow MFC as shown in Figure 1 was designed to hold the merits and avoid the corresponding drawbacks of the co-flow and counter flow MFCs.
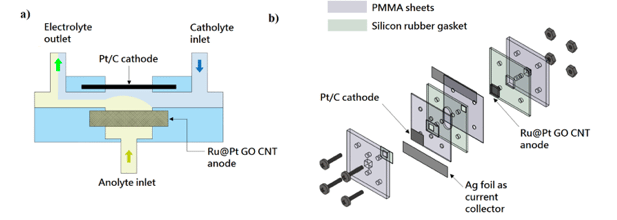
Figure 1, (a) schematic diagram of the orthogonal flow MFC and (b) exploded view of the MFC construction. Reproduced with permission from Elsevier (Applied Energy 217 (2018) 258-265).
The well-known Pt catalyst was replaced by Ru rich core and Pt-rich shell to lower the catalyst cost and increase the catalyst performance and stability at the same time. The core-shell nanoparticles produced by the two-step polyol process were less than 3nm, which were grown on the graphene aerogel. To increase the electrode conductivity, multi-walled carbon nanotubes (CNT) were added to form a composite. After freeze-drying, the electrode can be fixed into the MFC directly for performance testing.
The higher the catalyst loading, the higher the MFC power output. However, doubling the precious noble metal catalyst does not double the power output. A balance between cost and cell performance shall be made in real applications. The MFC performance could be enhanced by the addition of CNT as mentioned, increasing the supporting electrolyte concentration could help to reduce the ionic resistance while annealing the electrode to remove the contaminants on the catalyst surface and increase the electrode conductivity could also help a lot.
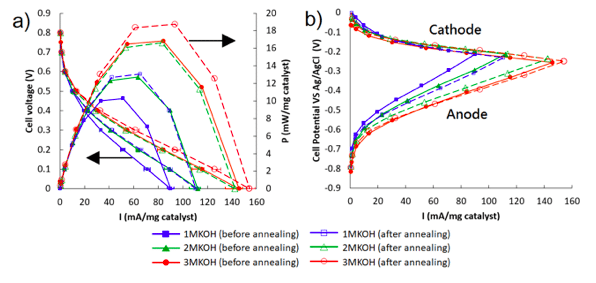
Figure 2, (a) Polarization curves of the MFC at different electrolyte concentration with 1M Methanol as fuel and the annealing effect to the MFC performance. (b) Corresponding single electrode performance of the MFC in (a). Reproduced with permission from Elsevier (Applied Energy 217 (2018) 258-265).
A maximum specific power of 13.1 mW/mg catalyst or 21.1mW/cm2 could be achieved when 1M KOH was used as supporting electrolyte, which out-performed the reported performance in the works of literature.
These findings are described in the article entitled Graphene-carbon nanotube composite aerogel with Ru@Pt nanoparticle as a porous electrode for direct methanol microfluidic fuel cell, recently published in the journal Applied Energy. This work was conducted by Y. H. Kwok, Y. F. Wang, Alpha C. H. Tsang, and Dennis Y. C. Leung from The University of Hong Kong.








