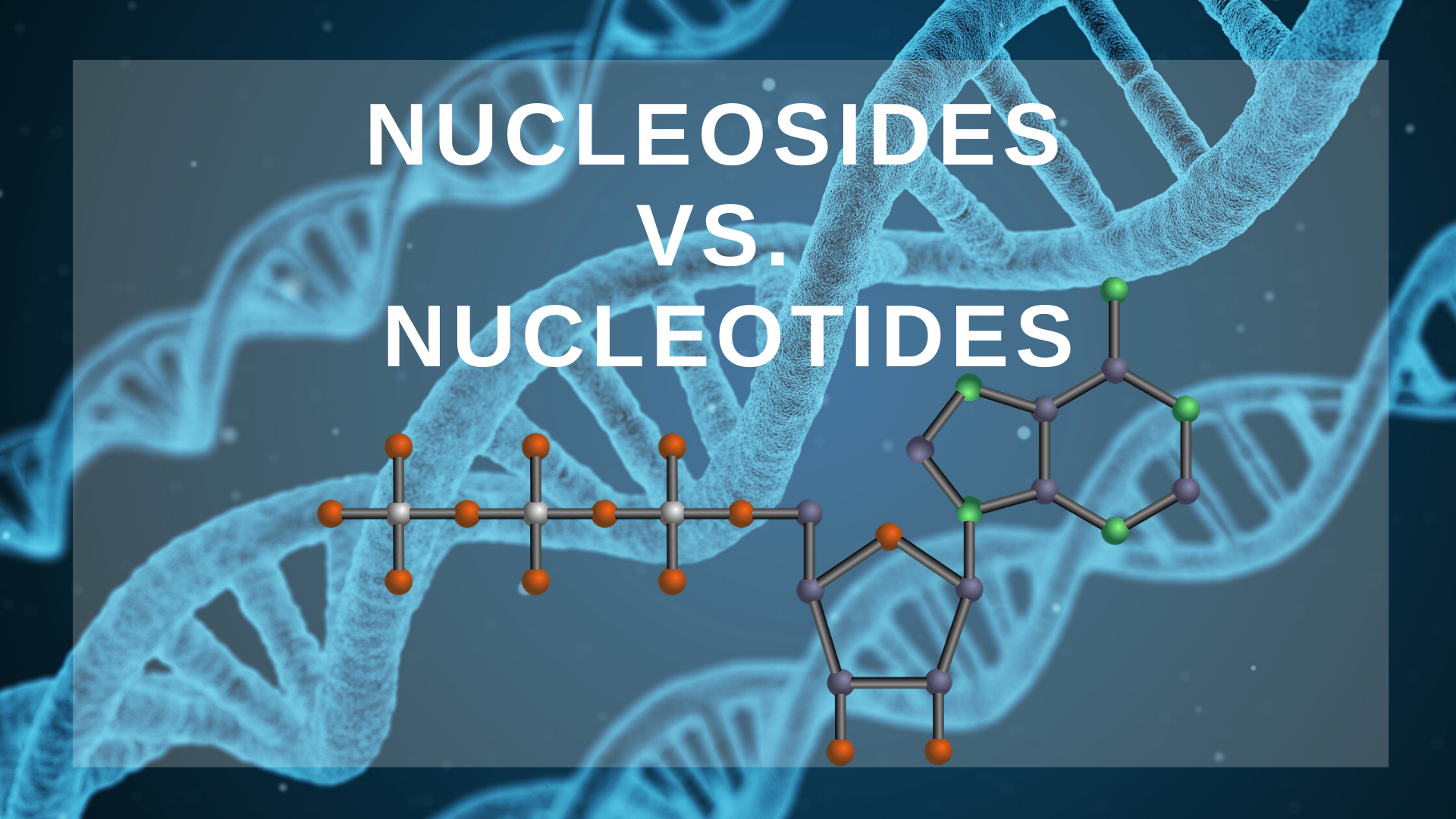
The difference between nucleosides vs. nucleotides involves the presence or absence of a phosphate group. A nucleoside consists of a nucleobase and a sugar (ribose or deoxyribose) whereas a nucleotide contains a nucleobase, a sugar, and one or several phosphate groups. Hence, the main difference is nucleotides have phosphate groups and nucleosides do not.
You’ve probably heard of DNA (Deoxyribonucleic Acid) before, along with RNA (Ribonucleic Acid). However, you may be less familiar with the compounds found within them, nucleosides and nucleotides. Nucleotides are molecules essential for creating life on Earth, yet they should not be confused with the similarly named nucleoside molecules. What’s the difference between nucleotides and nucleosides exactly?
“DNA is like a computer program, but far, far more advanced than any software ever created.” — Bill Gates
The short answer is that nucleotides are just nucleosides with a phosphate group added to them, and nucleosides themselves are just sugars linked to the nitrogenous bases that make up DNA and RNA. It’s important to put this answer in context though, so let’s take a closer look at the differences between nucleotides and nucleosides.
Before looking at nucleotides and nucleosides, let’s examine the bases that comprise DNA and RNA. DNA and RNA are made up of nitrogen bases also called nitrogenous bases. These nitrogen bases are divided into two different classes, pyrimidines, and purines. Pyrimidine is a compound made out of a single organic ring, while purines have a double ring structure made out of a pyrimidine ring combined with an imidazole ring.
The Five Nitrogenous Bases
There are five nitrogenous bases used to compose DNA and RNA: adenine, guanine, thymine, cytosine, and uracil. Each of the nitrogenous bases has a complementary base, so the two will only ever pair together. Adenine pairs with thymine, while guanine pairs with cytosine. The exception to this is uracil. DNA has adenine, guanine, thymine, and cytosine in it, but RNA lacks thymine and it bonds with adenine instead. Adenine is a purine, along with guanine. The other bases are pyrimidines.
The five nitrogenous bases bond with five-carbon sugars to create nucleosides. The names given to the nitrogenous bases paired with sugars are fairly close to the names of the bases themselves. An adenine base bonded with a five-carbon sugar is called adenosine when in the RNA and Deoxyadenosine when in the DNA.
The Role of Nucleosides
The cells in a body can gain nucleosides to use through the process of synthesis, but they can also be gained simply by ingesting nucleotides in food. Nucleotides will be broken down into nucleosides and phosphate by an enzyme referred to as nucleotidase. Nucleosides have use in the medical field, where they can function as antiviral or anti-cancer devices. In fact, the major cause of cancer is mutated and malfunctioning nucleotides.
“At the deepest level, all living things that have ever been looked at have the same DNA code. And many of the same genes.” — Richard Dawkins
Nucleosides go through the process of phosphorylation, bonding with phosphorous to create a nitrogenous base paired with sugar and phosphate, and a nucleotide is created. These nucleotides now have the famous sugar-phosphate backbone of DNA and function as the molecular building blocks for both RNA and DNA.
The Role of Nucleotides
The names given to nucleotides reflect the nitrogenous bases that make them up. For instance, one example of a nucleotide is adenosine triphosphate. The adenosine part of the name references adenine, while the triphosphate part of the name reflects how many phosphate residues the molecule has. Nucleotides can have up to three phosphate groups bonded to them, and they are referred to as monophosphate, diphosphates, and triphosphates respectively.
Nucleotides can be synthesized through a number of different methods. Synthesis of nucleotides in living cells, in vivo synthesis, can be done from the building blocks of bases and sugars, or they can be salvaged from other nucleotides ingested in food. Enzymes break down old nucleotides, freeing their parts up for the synthesis of new nucleotides. The liver is the organ responsible for synthesizing nucleotides.
An example of a specific nucleotide is adenosine monophosphate, which is not only used to create RNA but also plays an important role in the synthesis of ATP. ATP is the molecule that carries energy, giving cells the ability to do their work.
Though the five nucleotides based on the nitrogenous bases are the most relevant and common for people to learn about, other types of nucleotides exist. Cyclic nucleotides, like cyclic AMP, are molecules which have a cyclic bond between the phosphate and sugar of the molecule. These molecules have a variety of functions, including functioning as messengers for hormones.
The sugar-phosphate backbone of nucleotides is necessary for the nucleotides to link together and form the chains that comprise DNA and RNA. Nucleosides cannot form chains for this reason. Chains of DNA are created by linking together the phosphate of one nucleotide to the carbon of another nucleotide. As mentioned, the sugar carbon atoms have five points. Point 1 is where they attach to the nitrogen bases, and point 5 is where they attach to the phosphate group. This simple system of linking can construct chains of DNA polymers millions of nucleotides long, meaning that this allows the five base pairs to encode a functionally limitless amount of information.
Differences Between DNA and RNA
As for the structures that nucleotides create, what are the differences between DNA and RNA?
As mentioned earlier, RNA has uracil instead of thymine. Another difference between DNA and RNA is that DNA uses the sugar deoxyribose to form its nucleotides, while RNA uses the sugar ribose. Deoxyribose has one less oxygen atom than ribose does, hence the name deoxyribose. The most notable difference between the two acids is that DNA is a double-stranded molecule while RNA is a single-stranded molecule. This means that the nucleotides which link together also link with a complementary nucleotide on the other side, forming the double-helix structure that DNA is known for.
“With DNA, the ability to find out a lot more with a lot less has increased our ability for identification.” — Patricia Cornwell
The effect of DNA’s double helix structure means that it can store genetic information over long periods of time, and even repair itself if part of the DNA chain is damaged. This makes it more resilient and stable in aversive conditions, like being in a high alkaline environment. DNA doesn’t degrade in high alkaline conditions, while RNA does.
Remember that nucleosides and nucleotides are integral parts of the process that turns the four base pairs of DNA into all the life found on Earth, and try not to confuse the two.









