Cellular respiration is the process by which cells convert nutrients into the energy that is used to power a variety of functions like transportation, locomotion, and the synthesization of macromolecules.
The job of cellular respiration is to form adenosine triphosphate, a molecule used for energy. How does this transition between nutrients and adenosine triphosphate, or ATP, take place? What are the steps of cellular respiration?
When you arise in the morning, think of what a precious privilege it is to be alive – to breathe, to think, to enjoy, to love. – Marcus Aurelius
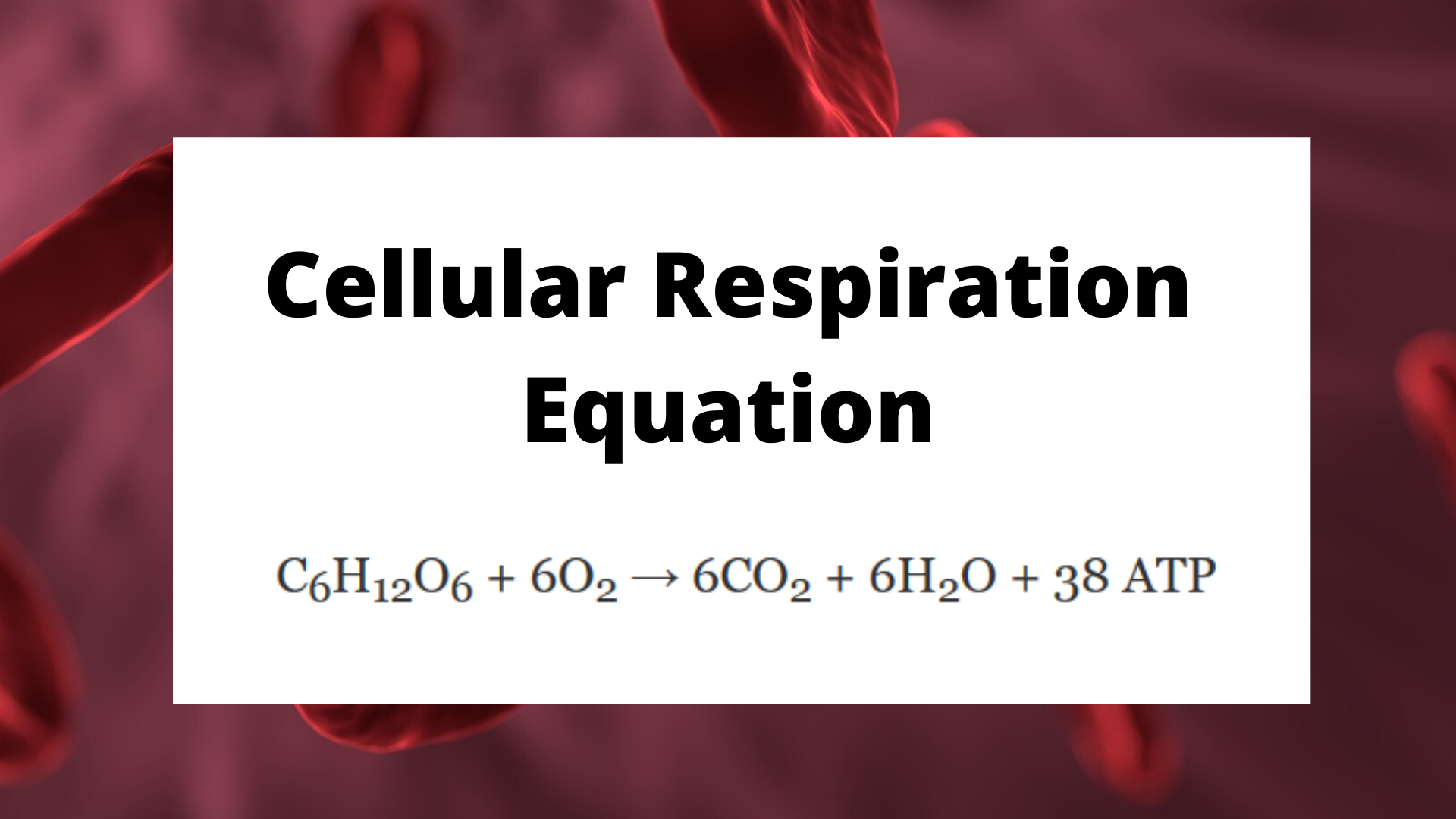
The Balanced Chemical Equation (Formula) For Cellular Respiration
The balanced equation (formula) that represents cellular respiration is:
C6H12O6 + 6O2 → 6CO2 + 6H2O + 38 ATP , or:

This formula could also be read as:
Glucose + oxygen –> water + carbon dioxide + energy
Essentially, this means that in cellular respiration glucose and oxygen are transformed to make water, carbon dioxide, and energy. Your body is utilizing the oxygen you breathe in as well as the food you eat to produce energy.
| 4 Stages Of Cellular Respiration |
| Glycolysis (The breakdown of glucose) |
| Link reaction |
| Krebs cycle |
| Electron transport chain, or ETC |
The oxygen you breathe in breaks down the sugars from your food and produces heat energy, similar to burning wood to release energy. In cellular respiration, the oxygen is used to break down the sugar, the energy of the sugar is released, and as a byproduct carbon dioxide is produced. The released energy is stored in your cells for later use.
“The cell never acts; it reacts.” — Ernst Haeckel
Some of the ATP used by cells comes directly from the reactions that caused the transformation of glucose. However, a large amount of ATP is produced later on during a phase of cellular respiration called oxidative phosphorylation. The process of aerobic respiration (aerobic meaning that it uses oxygen) is divided into four separate steps. Oxidative phosphorylation can be thought of as the final step of the cellular respiration process.
| Cellular Respiration Reactants | Cellular Respiration Products |
| Oxygen (6O2) | Water (6H2O) |
| Glucose (C6H12O6) | Carbon Dioxide (6CO2) |
| Energy (38 ATP) |
The Stages of Cellular Respiration
The four stages of cellular respiration are glycolysis, pyruvate oxidation, the citric acid cycle, and oxidative phosphorylation.
Glycolysis is the step where glucose is converted into other molecules through a number of chemical transformations. It takes place in the cytosol of cells, and it can actually function with or without oxygen. Glucose is a sugar with six carbons, and in aerobic respiration, glucose is converted into two pyruvate molecules. When the molecules of pyruvate are oxidized they produce two NADH, which helps carry electrons to other reactions, as well as two molecules of ATP.
Pyruvate oxidation happens when the pyruvate enters the mitochondrial matrix, which is the innermost part of the mitochondria (the structure of cells that produces energy). In the mitochondrial matrix, the pyruvate is bonded with Coenzyme A, and transformed into a two-carbon molecule. This new structure is known as “acetyl CoA”. This process generates NADH and releases carbon dioxide.
The citric acid cycle (also known as the Krebs cycle or the tricarboxylic acid cycle) is where the acetyl CoA that was produced in the last step is combined with a molecule of oxaloacetic acid. This forms a molecule of citric acid, which undergoes a complex cycle of reactions. The final step in this cycle creates a molecule of oxaloacetic acid so the cycle can start again. During the cycle, carbon dioxide is released and NADH, FADH2, and ATP are produced. The electrons in NADH and FADH2 are sent to the electron transport chain.
During the oxidative phosphorylation step, the molecules of NADH and FADH2 that were created during the other steps drop their electrons in the electron transport chain. This means that they are no longer loaded with electrons, reducing down to their simpler forms, NAD+, and FAD. The electrons then move across the transport chain, which releases energy. This process pushes protons out of the mitochondrial matrix, which forms a gradient. The protons return to the matrix via an enzyme called ATP synthase, which as it sounds, makes ATP. Finally, the electron transport chain ends when oxygen accepts the electrons and bonds with protons, forming water.
How Much ATP Is Made?
How much ATP is generated by this process overall? Most ATP is actually generated through the proton gradient that is created in the inner mitochondrial matrix. Oxidative phosphorylation could generate around 26-28 units of ATP, and substrate phosphorylation could generate around 4 to 6 more. However, preparation for glycolysis also consumes a bit of ATP, meaning that in actuality the total yield for the process is likely to be around 30 units of ATP.
Anaerobic Respiration and Fermentation
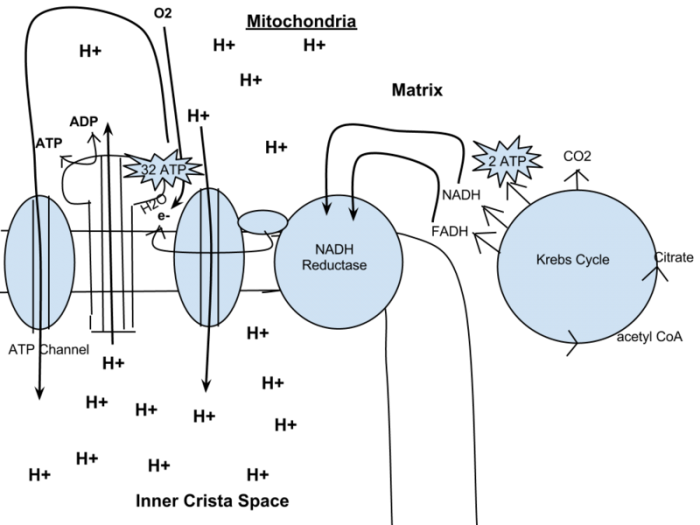
Figure representing cellular respiration in the organelles called mitochondria. “Respiration diagram” by Bio4lyfe via Wikimedia Commons, CC-BY-SA 3.0
All of the above processes assume that enough oxygen is available for the process of aerobic respiration. If oxygen is not available, what will happen instead is “anaerobic respiration,” a form of which is fermentation? Aerobic respiration is vastly more efficient than anaerobic respiration, producing around 18 times the amount of energy than fermentation does. By contrast, the process of fermentation will only produce a little ATP (2 units) and sometimes lactic acid.
During fermentation, the ATP is only extracted via the glycolysis pathway. The pyruvate that is created in glycolysis does not continue to go through the rest of the process, skipping oxidation and the citric acid cycle. It also doesn’t go through the electron transport chain. Due to the fact that the electron transport chain isn’t functioning, NADH cannot drop its electrons there and reduce to NAD+.
“A cell is regarded as the true biological atom.” — George Henry Lewes
There are instead a few extra reactions in fermentation that exist to create NAD+ from the NADH that was produced via glycolysis. This is accomplished by letting NADH use an organic molecule like pyruvate to drop the electrons it is carrying, and the ensured supply of NAD+ means that glycolysis can keep functioning.
Certain cells can do what is known as “lactic acid fermentation.” This process has NADH transfer any electrons it has to pyruvate molecules, meaning that lactate is generated as a byproduct. The kinds of bacteria that create yogurt carry out this process of lactic acid fermentation, but so do cells in your body. Your red blood cells and muscle cells can do lactic acid fermentation. In the case of your red blood cells, it is necessary for them to be able to do lactic acid fermentation because they don’t have mitochondria and thus cannot perform aerobic cellular respiration.
Your muscle cells will only do lactic acid fermentation when under strain after they have been used to exercise very hard and the oxygen that was present has already been used up. The lactic acid that is created by the muscle cells is then carried to the liver by the bloodstream. In the liver, it is converted back in pyruvate to be handled normally by cellular respiration.
The ability of cells to respirate and create energy is what allows them to carry out complex tasks. Thanks to this ability, cells can form a wide variety of organisms we see in the world.









