
Ammonia is a chemical compound that has the formula NH3, being made out of one nitrogen atom and three hydrogen atoms. The molar mass of NH3 (ammonia) is 17.031 g per mole. The chemical properties of ammonia include ammonia having high stability, being combustible in air, and forming nitric oxide when combined with a platinum-rhodium catalyst at approximately 800°C.
What’s A Mole?
The molar mass of a chemical substance is the mass which is possessed by a single mole of that substance. In order to understand why the molar mass of a substance is important, we will first need to define a mole. A mole is a unit of measurement for chemical substances, and it allows chemists or other scientists to determine how many elementary entities (molecules or atoms) are within a certain amount of a chemical substance. The number of elementary entities in one mole is equivalent to the number of atoms in 12 g of carbon 12. This number is referred to as Avogadro’s number, which is 6.022×10^23. This means that there are 6.022×10 to the 23rd elementary entities found in one mole.
So why is the mole important in chemistry? It is important to have a unit of measurement that represents a large number of elementary entities, because when chemical changes occur they involve billions of atoms being rearranged. These billions of atoms can’t realistically be visualized or represented in a timely fashion, yet scientists still have to have a method of representing the entire quantity of atoms. For this reason, moles are used to compare the weights of substances with the number of atoms, and weight can be empirically quantified rather simply in comparison to number of atoms.
When doing computations involving two different chemicals, using Avogadro’s number and moles is necessary. Utilizing Avogadro’s number is critical to understanding both how the molecules combine and the nature of their interactions. Let’s examine how two atoms of hydrogen create one molecule of water when they are combined with one oxygen atom. Remember that one mole of H2O is made out of two moles of hydrogen and one mole of oxygen, so this can be represented as 1 mole of H2O = 2 × 6.022×1023 of Hydrogen + 6.022×1023 of Oxygen.
One notable property of Avogadro’s number is that the substance’s molecular weight will be equal to the molar mass of the given substance. As an example, a mole of water will weigh 18.015 g, while a molecule of water’s mean weight is 18.015 atomic mass units (AMU).
Summing Up:
The molecular weight of a substance is equal to The mass that one mole of a substance possesses. As an example, one mole of water weighs approximately 18.015 g, and 18.015 atomic mass units is the mean molecular weight of water.
What Is Molar Mass And Why Is It Important?

Photo: PublicDomainPictures, URL: https://pixabay.com/photos/lab-research-chemistry-test-217043/, CC0
Knowing the molar mass of a substance is important because molar mass bridges together the number of moles in a sample of the substance and the mass of the material, so without molar mass, it wouldn’t be possible to directly measure the number of moles.
Every atom or ion has its own mass, and not only that but there’s also a definite mass for every mole of a substance. When you have a pure element, the mass that one mole of that element’s atoms possesses in grams is equivalent to the atomic mass of that element. This is true whether or not the calculation is being done in grams per mole or in atomic mass units.
The molar mass of a substance is equal to the mass of the substance divided by the quantity of that substance. Grams per mole is how molar mass is measured. As an example, the molar mass of titanium is 47.88 g per mole or 47.8 AMU. This means that there are 6.022×10 to the 23rd titanium atoms in 47.88 g of titanium.
The atomic mass in grams per mole is also equivalent to the characteristic molar mass of that element. The molar mass of a substance can be determined through alternate methods such as multiplying the molar mass constant which is 1 g per mole by the atomic mass in AMU. You’ll end up summing the atomic mass of the various constituent atoms In order to determine the molar mass of a compound that has multiple kinds of atoms. To determine the molar mass of NaCl, for instance, you need to find both the atomic mass of chlorine and the atomic mass of sodium. Sodium’s atomic mass is 22.99 g per mole while chlorine’s atomic mass is 35.45 g per mole. You simply combine the two masses to get 58.44 g per mole, the molar mass of NaCl.
Ammonia’s Structure
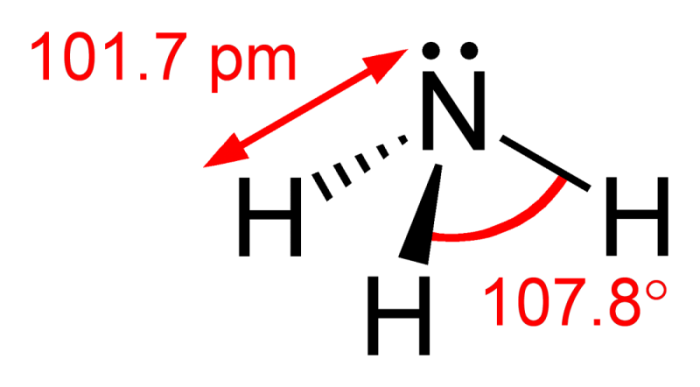
Note the angles of the bonds within ammonia. Photo: By Ben Mills – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=6628922
Examining ammonia’s structure reveals that it is a covalent molecule. The overlapping of three SP3 hybrid orbitals, as well as the three hydrogen orbitals, forms ammonia. There is another SP3 hybrid orbital in the molecule, but this last orbital only has a lone pair. This structure means that the ammonia molecule has a triangular middle shape to it. The angle of the hydrogen-nitrogen-hydrogen bond is 107.3°. The bond angle of the H and H bond is just a little less than the 109° tetrahedral angle. The angle of the H and H bonds is a result of the fact that the lone pair bond frequently pushes the NH bond inwards a little. In both solid and liquid states hydrogen bonds are responsible for the association of ammonia.
Physical Properties Of Ammonia
Ammonia is colorless, however, unlike some other colorless gases it is not odorless. In fact, ammonia has a very pungent odor, and when inhaled it can cause coughing, choking and the watering of the eyes. It is also said to have a soapy/alkaline taste. Ammonia is lighter than air, and so regular air will displace it as it moves downward and the ammonia will congregate in a high area. Ammonia is water soluble, and water can dissolve around 1300 volumes of ammonia gas into it. Thanks to the high water solubility of ammonia it can’t congregate over a body of water. Ammonia liquifies at room temperature quite easily when around 10 atmospheric pressure is applied. Liquid ammonia will freeze at around 195.3 K (-77.8°C), and it produces a solid crystalline, white colored structure. At one atmospheric pressure, liquid ammonia boils at 239.6 K (- 33.5°C). Ammonia is often used as a refrigerant thanks to its high latent heat of vaporization.
Chemical Properties Of Ammonia
One of ammonia’s primary chemical properties is thermal stability. Ammonia is a very stable chemical compound, but it can decompose into nitrogen and hydrogen by using a heated metallic catalyst or by passing an electrical discharge through the compound. Another chemical property of ammonia is the fact that it is combustible in air, and in an oxygen environment, it will burn. Nitric oxide is produced when air and ammonia are passed through a rhodium-platinum catalyst at approximately 800°C.
Ammonia is miscible when combined with water. When water is boiled the ammonia can be removed from it. Ammonia will only burn readily in mixtures of approximately 15 to 25% air, so there it must be in very narrow air mixtures. However, when it does burn it makes a yellowish-green flame. The combustion of ammonia produces hydrogen and water through the exothermic reaction.
Ammonia molecules have an interesting relationship with other molecules. Ammonia molecules will very frequently donate their lone electron pairs to other molecules. This property means that they act similarly to a Lewis base. Molecules of NH3 ionize in accordance with the donation reaction when it is placed in an aqueous solution. The reaction that makes ammonia donate its lone pair of electrons has a constant equilibrium of 1.8 x 10^-5 at 298K.
Uses For Ammonia
Ammonia molecule. The molecule is made out of one nitrogen atom (blue) and three hydrogen atoms (gray). Photo: By Ben Mills – Own work, https://commons.wikimedia.org/w/index.php?curid=3958453, Public Domain,
One of the primary uses for ammonia is as an ingredient in fertilizers. When ammonia is used to create fertilizers and that fertilizer is used on soil, the yield of crops like wheat increases. Ammonia is also used in the creation of many nitrogen-containing compounds. Almost all nitrogen-containing synthetic compounds are created using ammonia as a catalyst/ingredient. Ammonia is also used to create amino acids, phenol hydrazine, and hydrogen cyanide.
Ammonia is also a primary ingredient in many household cleaners. Ammonium hydroxide is NH3 dissolved into water, and it cleans surfaces without leaving many streaks behind. Ammonia-based cleaners are effective at cleaning surfaces covered in grime. Cleaners with ammonia range somewhere from 5 to 10% ammonia by weight. Solutions that are comprised of approximately 16 to 25% ammonia are often provided to microorganisms as a source of nitrogen when used by the fermentation industry. Ammonia solutions are also frequently used as an antimicrobial agent to reduce the contamination of beef.









