
The organophosphorus pesticides (OPs) are synthetic esters, amides, and thiol derivative of the phosphoric, phosphorotioic, and phosphonothioic acids (Fig. 1)
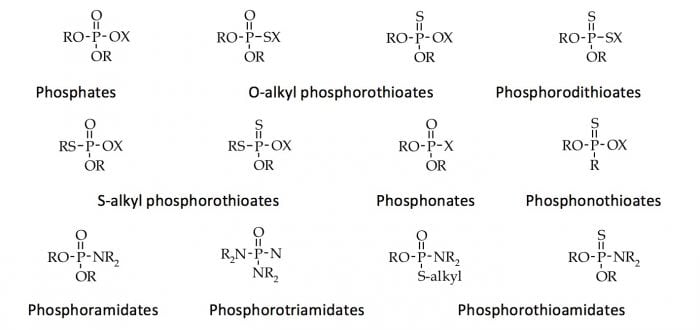
Fig. 1. Chemical structure of the organophosphorus pesticides. Republished from Open Access intechopen.com.
They are the most largely used pesticides (Fig. 2) during the last decades because of their high efficiency and low persistence in the environment.
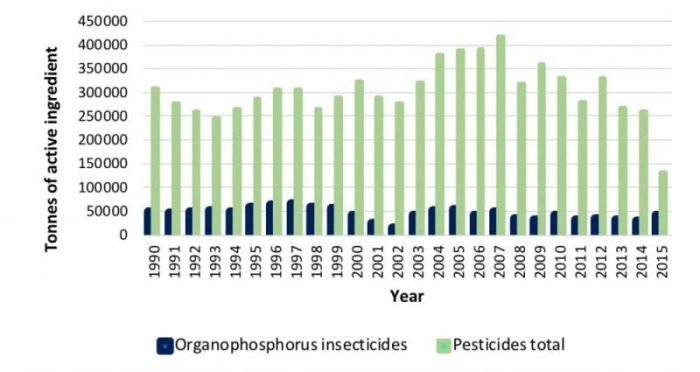
Fig. 2. Pesticides consumption, according to FAOSTAT. Margarita Stoytcheva
The increased and indiscriminate OPs application and the related public and environmental concerns implicated the development of sensitive, selective, specific, rapid, simple, and economic analytical methods for their monitoring, such as the electrochemical (bio)sensors-based techniques.
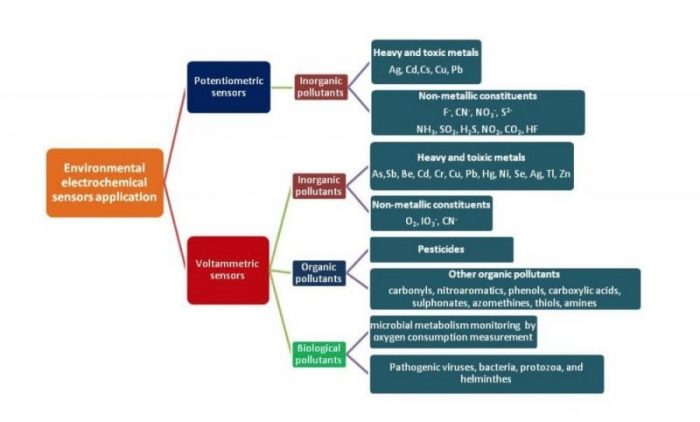
Fig. 3. Environmental electrochemical sensors application. Margarita Stoytcheva
The electrochemical biosensors for OPs determination are mainly amperometric including the enzyme acetylcholinesterase (Ach) or the enzyme organophosphorus hydrolase (OPH) as biological recognition element. The thiocholine oxidation current, generated upon the Ach-catalyzed hydrolysis of acylthiocholine is registered as an analytical signal of the Ach-based sensors. It decreases in the presence of organophosphorus pesticides, because of the provoked enzyme inhibition.
The Ach-based sensors are very sensitive, but they are not specific to the individual organophosphorus compounds. In contrast, the OPH-based sensors, although less sensitive, allow the selective determination of the group of the OPs with electroactive nitrophenyl leaving groups, such as paraoxon, parathion, etc., and their distinction in the presence of other OPs. The sensor response is the oxidation current of the nitrophenols produced upon the OPH-catalyzed OPs hydrolysis.
The selective determination of the great number of OPs with phenolic leaving groups, which are not electroactive still remains a challenge. A successful approach to overcome this problem was suggested by Stoytcheva et al. (Electroanalysis, 2017, 29, 2526-253) applying a bi-enzyme amperometric sensor.
The biosensing platform was created by simultaneous enzymes co-immobilization by controllable and reproducible one-step electrodeposition onto the surface of a glassy carbon electrode of chitosan-entrapped carboxylated multi-walled carbon nanotubes, OPH, and horseradish peroxidase (HRP). The functioning of this approach is illustrated by the following reactions, selecting prothiofos as an example (Fig. 4).
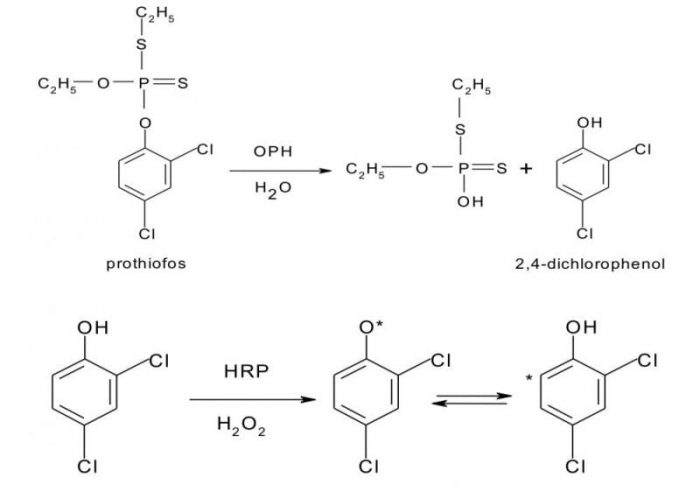
Fig. 4. Reaction sequence illustrating the principle of the prothiofos determination by applying a bi-enzyme (OPH-HRP) amperometric sensor. Margarita Stoytcheva
As shown in Fig. 4, the reaction sequence starts with the OPH-catalyzed hydrolysis of prothiofos, which results in 2,4-dichlorophenol (DCP) production. The second reaction step, i.e. the HRP-catalyzed oxidation of the generated in the enzymatic layer DCP using H2O2 as an oxidant, leads to electroactive phenolic radicals formation. The analytical signal of the bi-enzyme sensor is the reduction current of the obtained radicals proportional to the DCP concentration in the presence of a constant amount of H2O2, as well as to the prothiofos concentration.
The developed bi-enzyme sensor allows the determination of the organophosphorus compounds with phenolic leaving groups (Fig. 5) excluding the interference of the nitrophenyl-substituted OPs.
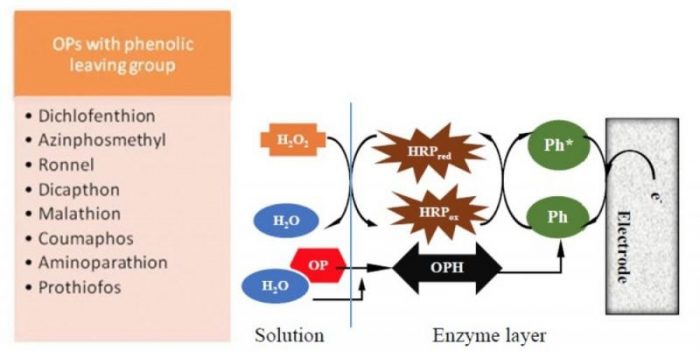
Fig. 5. Determination of OPs with phenolic leaving groups by applying a bi-enzyme (OPH-HRP) amperometric sensor. Margarita Stoytcheva
These findings are described in the article entitled Bi-enzyme Electrochemical Sensor for Selective Determination of Organophosphorus Pesticides with Phenolic Leaving Groups, recently published in the journal Electroanalysis, 2017, 29, 2526-532.
This work was led by Margarita Stoytcheva, Roumen Zlatev and Gisela Montero from the Instituto de Ingeniería of the Universidad Autonoma de Baja California at Mexicali, Mexico, Zdravka Velkova from the Medical University of Plovdiv, Bulgaria, and Velizar Gochev form Plovdiv University, Bulgaria.








