
Muriatic acid is the historical name for hydrochloric acid, an aqueous solution of hydrogen chloride (HCl) and water (H2O). Hydrochloric acid is one of the simplest acids as it results from the dissociation of a diatomic molecule HCl. It is also one the strongest acids; a 0.1 molar solution of hydrochloric acid has a pH of 1.1. Hydrochloric acid is strong because the hydrogen chloride molecules completely dissociate into hydrogen ions (H+) and chlorine (Cl–) ions.
Hydrochloric acid is a strong reagent that has many industrial uses, specifically in treating metals. Steel is treated with hydrochloric acid to remove any rust and oxidization before processing. Hydrochloric acid is also used to produce several organic and inorganic compounds and as a means of dissolving limestone and other carbonate substances. Hydrochloric acid is one of the main ingredients of gastric acid and is found in the stomach of most vertebrates. In high concentrations, hydrochloric acid is corrosive and can cause burns to skin and mucous membranes.
What Is An Acid?
Acid is the name for a chemical system that forms when a molecule or ion dissociates in water and donates a hydrogen ion (H+). The strength of an acid is determined by the concentration of hydrogen ions in the solution; the higher the concentration, the stronger the acid. Acids are contrasted with bases, solutions with a low concentration of H+ ions. Acidic solutions tend to have a sour taste, turn litmus paper red, and will react with bases and certain metals to form ionic salts.
The definition and behavior of acids and bases arise from the acid-base behavior of water itself. In any given sample of water, hydrogen ions are spontaneously generated via a process called autoionization. During autoionization, water molecules randomly dissociate into hydrogen ions and hydroxide ion (OH−) according to the reaction:
H2O ↔ H+ (aq) + OH− (aq)
The letter in parentheses just means that the ions are in a solution of water. As the equation indicates, autoionization creates equal amounts of H+ and OH− ions. Although chemists write H+ (aq) to signify the dissociation of hydrogen ions in water, hydrogen ions do not exist in lone proton form. They instead attach to a neighboring water molecule to form a hydronium ion (H3O+). Scientists write H+ (aq) as a shorthand convention.
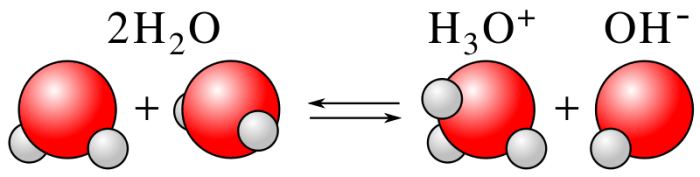
The autoionization of water. Credit: WikiCommons CC0 1.0
In a one liter sample of pure water, the concentration of hydrogen ions produced by autoionization is 1.0×10−7 M. Solutions are classified as acids or bases dependent on their concentration of hydrogen ions relative to pure water. Acidic solutions have a hydrogen ion concentration greater than 1.0×10−7 M and basic solutions have a concentration lower than 1.0×10−7 M.
This is where we get the traditional pH scale for measuring the acidity of a solution. On the pH scale (“pH” means “potential hydrogen”), pure water is given a value of 7. More acidic solutions have a pH less than 7 and more basic solutions have a pH greater than 7. Specifically, the pH of a solution is related to the concentration of hydrogen ions by the formula:
pH = −log10[H+]
where [H+] is the concentration of hydrogen ions. The pH scale is logarithmic, meaning that solutions that differ by 1 pH differ 10-fold in the concentration of H+ ions. The pH of blood and cellular cytoplasm in the human body is between 6.8–7.2—almost exactly neutral.

Orange juice is a common acidic solution. Credit: Pixabay CC0 1.0
With this understanding, we can define an acid as a substance that increases the concentration of H+ ions when dissolved in water. Most acids do this by donating a single proton, dissociating into a hydrogen ion and a conjugate base. Bases, in turn, as substances that decrease the concentration of H+ ion. Most bases do this by creating an OH− ion or some other ion that can bond with hydrogen ions.
Properties Of Hydrochloric Acid
The stronger an acid, the more completely it dissociates in a solution of water. Hydrochloric acid is produced by dissolving gaseous hydrogen chloride (HCl) in water. When dissolved, HCl dissociates completely into H+ and Cl− ions according to the reaction:
HCl + H2O → H3O+ + Cl−
In this context, “strong” just means how well the acid dissolves, not how dangerous or corrosive it is. In fact, hydrochloric acid is one of the least hazardous strong acids to handle as it contains the non-toxic and non-reactive chlorine ion Cl−. Other weaker acids are much more dangerous. For instance, sulfuric acid (H2SO4) is weaker than hydrochloric acid, but sulfuric acid can cause serious burns even in moderated concentrations. Highly concentrated amounts of hydrochloric acid can still be dangerous though, particularly the fumes which can irritate the lungs.
Hydrogen chloride. Credit: WikiCommons CC0 1.0
The freezing, melting and boiling points of hydrochloric acid depend on the concentration of dissolved HCl. Low concentration solutions have phase change points similar to pure water, but higher concentrated solutions will create fumes of hydrochloric acid at room temperature.
Production Of Hydrochloric Acid
Hydrochloric acid is prepared by dissolving hydrogen chloride in water. Hydrogen chloride can be made a number of ways so preparations of hydrochloric acid have many precursors. Hydrogen chloride can be directly synthesized by the electrolysis of salt water. Electrolysis of salt water creates hydrogen and chlorine gas, which can then be combined into hydrogen chloride by being exposed to UV rays.
Most industrial amounts of hydrochloric acid are produced in tandem with the chlorination and fluoridation of organic hydrocarbons. The hydrogens on the hydrocarbons are replaced with chlorine atoms, and the freed hydrogens are combined with spare chlorine atoms to form hydrogen chloride, which directly dissolves in the water used in the reaction. This process is used to create industrial strength hydrochloric acid with concentrations of up to 38%. Higher concentrations are possible but the resulting fumes are difficult to contain and require specialized equipment to handle and store.
Hydrochloric acid can also be produced directly in the laboratory by the reaction of sodium chloride and sulfuric acid. Sodium chloride and sulfuric acid react according to the formula:
NaCl + H2SO4 → NaHSO4 + HCl
The produced hydrogen gas can be captured and dissolved in water.
Uses Of Hydrochloric Acid
One of the most prominent uses of hydrochloric acid is in steel production. Pre-processed steel is treated with a solution of hydrochloric acid to remove any rust formed from an iron oxide, according to the reaction:
Fe2O3 + Fe + 6HCl → 3FeCl2 + 3H2O
The hydrochloric acid removes rust from the steel, producing ferrous chloride and water. The spent acid is then reconverted back into hydrochloric acid by the introduction of molecular oxygen:
4FeCl2 + 4H2O + O2 → 8HCl + 2Fe2O3
The introduction of molecular oxygen essentially removes the rust from the hydrochloric acid, allowing it to be used again to treat steel. The iron(III) oxide produced form this regeneration process is itself a valuable reagent and is used in the production of thermite.
Hydrochloric acid is also used to dissolve limestone and other minerals. Limestone is calcium carbonate (CaCO3), an alkali salt, so hydrochloric acid will dissolve it according to the reaction:
CaCO3 + 2HCl → CaCl2 + CO2 + H2O
Hydrochloric acid will dissolve calcium carbonate to produce calcium chloride and carbon dioxide gas. This process is used to dissolve limestone deposits and mineral build up from hard water.
In general, the strong acid properties of hydrochloric acid make it useful because it is a predictable reagent to work with. Hydrochloric acid reacts according to the simplest acid-base reaction rules, so it can be exploited to make a number of organic and inorganic salts, metal oxides, chlorides. This versatility makes hydrochloric acid useful across industries.
Hydrochloric acid is also one of the main constituents of gastric acid. Hydrochloric acid in gastric acid dissolves organic matter, freeing up its nutrients to be processed in the intestines. The stomach protects itself from the corrosive effects of hydrochloric acid by secreting a thick mucus lining containing sodium bicarbonate. The sodium bicarbonate neutralizes the hydrochloric acid and acts as a protective barrier.
History Of Hydrochloric Acid
Hydrochloric acid has served as an important chemical reagent throughout history. There is some evidence that the ancient Egyptians and Babylonians had access to the compound, but the first explicit identification of hydrochloric acid as a unique substance was in 800 AD by the Persian chemist/alchemist Jabir ibn Hayyan. Originally, hydrochloric acid was produced via a reaction involving rock salt (a crystallized rock formation of NaCl) and “green vitriol” (iron (II) sulfate). Other historical names for hydrochloric acid include “spirit of salt,” “acidium salis,” and “salt acid.”
During the Middle Ages, “aqua regia,” a mixture of hydrochloric and nitric acids, was an alchemical ingredient used to dissolve gold and platinum, two normally inert metals. This ability to dissolve noble metals gives aqua regia its name, which translates to “king’s water.”
Hydrochloric acid as we understand it today was first produced in the 17th century by the German chemist Johann Glauber. Glauber first produced hydrochloric acid via the reaction of sodium chloride and sulfuric acid, a method called the Mannheim process which is still used today.








