
Amino acids are compounds which contain the functional groups amine and carboxyl, and are composed of the elements hydrogen, oxygen, nitrogen, and carbon primarily, though some other elements find their way in. Eukaryotic organisms have 21 essential amino acids found within their cells, and this includes in the human body.
Let’s go over the different types of amino acids, including the 21 essential amino acids and some nonessential amino acids.
The Structure Of Amino Acids
Amino acids are used by the ribosomes to create proteins. While the exact structure will vary between specific amino acids, in general, amino acids have a carbon atom located next to a carboxyl group and this formation is referred to as the a-carbon (alpha carbon) structure. Those that contain an amino group linked to this alpha carbon are dubbed alpha-amino acids.

pKa’s are related to the pH scale and different amino acids can have different levels of acidity. Photo: By OpenStax College – Anatomy & Physiology, Connexions Web site. https://openstax.org/books/anatomy-and-physiology/pages/1-introduction, Jun 19, 2013., CC BY 3.0, https://commons.wikimedia.org/w/index.php?curid=30131151
Amino acids are divided into separate groups. Amino acids are grouped partially based off of their acid dissociation constant, usually represented as pKa. A group’s pKa is related to the pH value of the acids, specifically, it’s the pH value where the number of molecules in the protonated group is equal to that of the un-protonated group of amino acids. The base amino group usually has a pKa value of around nine or 10. In contrast, the acidic alpha carboxyl group has pKa values which are around two.
At a pH of between 7 to 7.4, free amino acids are usually what are called “zwitterions” (translated from German as “hybrid ions”) or dipolar ions. Every protein and any free amino acid will have a pH at which it becomes a zwitterion. Put another way, all proteins and amino acids have a change in their pH value that causes it to have an equal number of negative charges and positive charges. The pH value where this equilibrium occurs is referred to as the isoelectric point.
When proteins and amino acids are dissolved in water they are mainly in their isoelectric form. The isoelectric point is when molecules have a net zero charge, though there is no pH value at which molecules have zero charge overall or a total absence of negative and positive charges. This means that proteins and amino acids will always be in the form of ions, and will always have group charges – positive or negative.
Different Amino Acid Groups
Common amino acids are grouped based off of the polarity of the amino acids, with polarity based on the side chain or R-group.
Group 1 Amino Acids
Group 1 amino acids include proline, phenylalanine, methionine, valine, leucine, glycine, alanine, tryptophan, isoleucine, and proline. These group 1 amino acids are hydrophobic in nature, meaning that they repel water. These proteins are capable of folding into a 3-D shape and enveloping the hydrophobic side chains within the interior of the protein.
Isoleucine has a two carbon atom within it and is just a modified molecule of regular leucine. Phenylalanine is made out of an alanine group and a phenyl group, as the name implies. Methionine is a protein which possesses a sulfur atom, and it is linked with translation or protein biosynthesis. Methionine is the amino acid which almost exclusively starts the translation process. Something unique about proline is that it’s the only amino acid amongst the standard amino acids that doesn’t have a free alpha carboxyl group and a free alpha amino.
Group 2 Amino Acids
While group 1 amino acids are nonpolar, group 2 amino acids are polar and uncharged. The group 3 amino acids include glutamine, tyrosine, serine, asparagine, cysteine, and threonine. Most of these amino acids have atoms which can create hydrogen bonds with water or other molecules. Tyrosine is a phenol derivative, and it possesses a hydroxyl group. Threonine and serine both have aliphatic hydroxyl groups. Cysteine has a sulfur atom, though unlike the sulfur atom found in the methionine, the sulfur of cysteine is chemically reactive. Both glutamine and asparagine have amide R groups.
Group 3 Amino Acids
Group 3 amino acids are acidic amino acids. There are only two amino acids in this group: glutamic acid and aspartic acid. Both of these acids have a side chain which contains a carboxylic acid. When the amino acids are at physiological pH and in an aqueous solution, the functional parts of the acids ionize. As a result, the two amino acids gain an overall charge of -1. The amino acids are referred to as glutamate and aspartate in their ionic forms.
Aspartate and glutamate’s side chains are capable of forming salt bridges or ionic bridges. These side chains are also capable of acting as hydrogen bond acceptors. Metalloproteins are proteins that bind metal ions together, and they have glutamate or aspartate side chains in their metal binding regions. Both glutamine and glutamate are involved in the metabolism of amino acids, and glutamate is a neurotransmitter found within the central nervous system.
Group 4 Amino Acids
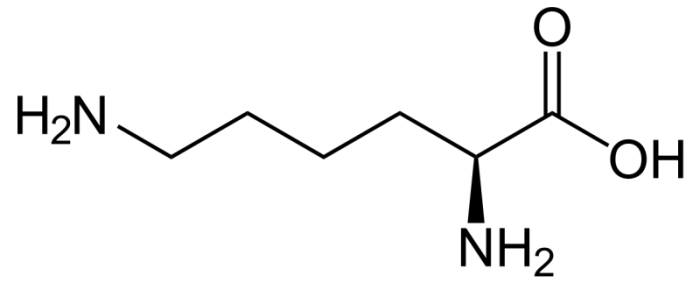
Chemical structure of the amino acid lysine. Photo: By NEUROtiker – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=1637097
Group 4 amino acids are the basic amino acids, and they include lysine, arginine, and histidine. The basic nature of the side chains means that they are capable of accepting protons. Arginine and lysine have a +1 overall charge when they are at physiological pH. Arginine has a guanidino group in its side chain and out of all the R-groups it is the most basic. Lysine and arginine are similar to glutamate and aspartate as they are capable of forming ionic bonds with their side chains.
Histidine has an imidazole side chain that lets it function as both base and acid catalysts when it is near the physiological pH. Because the other standard amino acids don’t have this ability, histidine is an amino acid which often serves as the active region for protein enzymes.
Proteinogenic Amino Acids
Amino acids which create proteins during the translation are known as proteinogenic amino acids. There are 22 genetically encoded amino acids of his nature, though eukaryotes only have 21 of these amino acids with 20 being part of their regular DNA code. Humans are capable of making 12 of these amino acids from various molecules, and these amino acids that can be metabolized are referred to as nonessential amino acids. The other nine amino acids have to be obtained by consuming other organisms, and they are referred to as the essential amino acids.
The 21 different amino acids that are directly produced through protein synthesis are:
Arginine, Histidine, Lysine, Aspartic Acid, Glutamic Acid, Serine, Threonine, Asparagine, Glutamine, Cystine, Selenocysteine, Glycine, Proline, Alanine, Isoleucine, Leucine, Methionine, Phenylalanine, Tryptophan, Tyrosine, Valine
The Essential Amino Acids
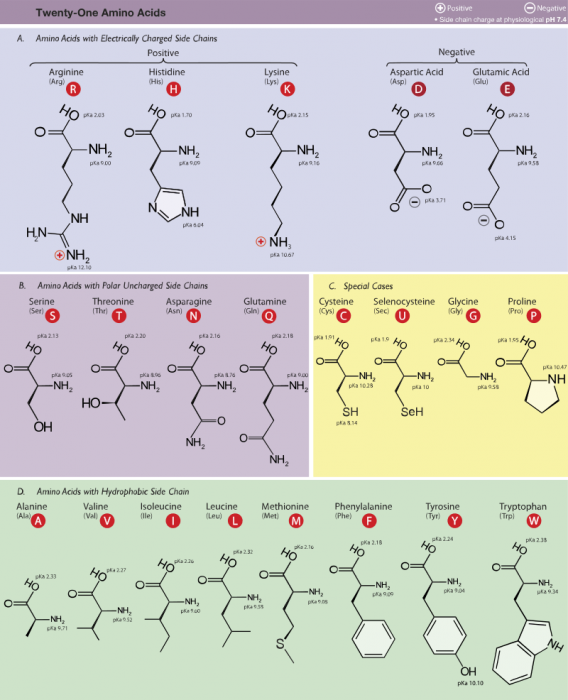
The 21 amino acids human cells use. Photo: By Dancojocari – Own workPrint It HereThis W3C-unspecified vector image was created with Adobe Illustrator.iThe source code of this SVG is valid., CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=9176441
The essential amino acids are valine, tryptophan, methionine, leucine, isoleucine, lysine, histidine, phenylalanine, threonine. These proteins must be gained by eating other organisms.
Phenylalanine is used to create norepinephrine, epinephrine, tyrosine, and dopamine. Valine is used to regenerate cells, produce energy, and prompt muscle growth. Threonine is involved in the production of elastin and collagen, which are both important parts of connective tissues and skin. Tryptophan is needed to regulate mood, sleep, and appetite. Methionine is another amino acid necessary for the growth of tissues. Methionine also lets the body absorb selenium and zinc.
Leucine is necessary for the regulation of blood sugar levels, the production of growth hormones, and for healing wounds, as it is necessary for both muscle repair and protein synthesis. Isoleucine is a modified version of leucine, and it is necessary for muscle metabolism (it’s also found in large concentrations in muscle tissue). Lysine is involved in the absorption of calcium, the production of various enzymes and hormones, and the synthesis of proteins. Lysine is also important for the immune system, the production of elastin and collagen, and energy production.
Histidine is necessary for the production of histamine. Histamine is required for many different neurological functions, as it is a neurotransmitter. Specifically, histidine is involved in the control of sleep-wake cycles, digestion, immune system responses, and sexual function. Histidine is also necessary for the maintenance of the myelin sheath, which insulates and protects nerve cells from degradation. Recent research implies that histidine can be produced by the body if it has an adequate supply of the eight other amino acids.
While these are the primary essential amino acids, there are a few amino acids that are essential only under certain conditions, such as if you are ill or under considerable stress and your body can’t otherwise meet the demands for these amino acids. This includes amino acids like arginine that have to be supplied through the diet in these circumstances. While humans need these 9 essential amino acids, the essential amino acids can vary between species.
Nonessential Amino Acids
The nonessential amino acids are histidine, tyrosine, arginine, serine, proline, glycine, glutamine, glutamate, cysteine, apartate, asparagine and alanine.
Some older textbooks will list only 20 amino acids in human cells. This is because the 21st amino acid, selenocysteine was only discovered fairly recently.








