In the broadest sense, nanomaterial is defined as a structure that is within the size of 1-1000 nanometers, i.e., 10-9 – 10-6 meters, in two or three dimensions. The nanoscale size of these materials holds numerous advantages over their bulk counterparts such as an increased surface-area-to-volume ratio along with unique physical, chemical, and optical properties.
These unique properties together with their small size make nanomaterials perfect candidates for biological application such as cell imaging. For example, silicon carbide nanowires become luminescent and can enter cancer cells, which enables them to track cells by fluorescence imaging (Figure 1).
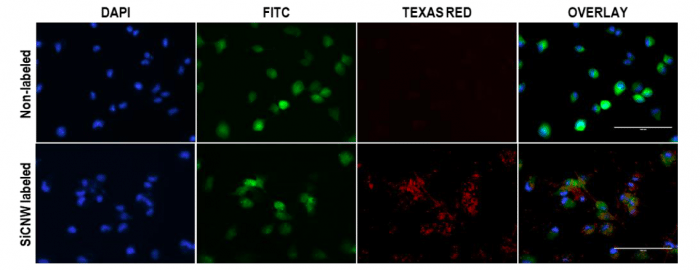
Figure 1. Cancer cell tracking with silicon carbide nanowires (SiCNW) by fluorescence imaging. Scale bars are 100 µm. Signal in TEXAS RED channel is due to the luminescence of silicon carbide nanowires. Signal in DAPI channel is from cell nucleus stained with NucBlue®. Signal in FITC channel is from green fluorescence proteins in the cancer cells. Republished with permission from Elsevier: https://doi.org/10.1016/j.biomaterials.2018.06.027
The medical application of nanomaterials, also known as nanomedicine, has been extensively reported over the last few decades[1, 2]. Existing applications of nanomaterials include targeted drug delivery, sustained drug release, highly sensitive imaging, and specific light- and heat-based therapies. For example, a nanoparticle that is made to specifically target a tumor can be loaded with an anti-cancer drug and be used for photothermal therapy. In other words, the drug is only released at a specific site as the nanoparticle carrier ablates the surrounding cells.
Nanomedicine shows great successful diagnostic and therapeutic outcomes in the lab setting. However, few nanomedicines make it to clinical trials, not to mention translation from bench to bedside. This is due to a limited understanding of the materials’ elusive toxicity.
Understanding the toxicity of nanomaterials is complicated because there are many factors to consider. Besides common factors like compositions and dosages, other reported factors include size, surface bond, synthetic methods, and shape[3]. Recently, Fang Chen et al. from Dr. Jokerst’s Bioimaging Lab at UCSD reported that the cytotoxicity of nanomaterials varies across cell lines. They showed that the silicon carbide nanowires were not toxic to MCF7 cancer cells, which confirms previous reports [4].
However, they found that silicon carbide nanowires negatively impacted multiple aspects of human mesenchymal stem cells’ function such as adhesion ability, cell proliferation, osteogenic differentiation, adipogenic differentiation, surface markers, and cytokine secretion. This finding provides important insight into learning about the cytotoxicity of nanomaterials before broadening their biological and/or medical applications.
Despite the toxicity of silicon carbide nanowires to human mesenchymal stem cells, the authors showed that two silicon carbide nanoparticles with varying sizes are compatible with human mesenchymal stem cells, showing minimal cytotoxic effects. This report indicates that materials with varying shapes — even if they are chemically identical — can significantly change a cell’s ability to survive and to function. This also further emphasizes the need for a rigorous understanding of toxicity.
These findings are described in the article entitled Cellular toxicity of silicon carbide nanomaterials as a function of morphology, recently published in the journal Biomaterials. This work was conducted by Fang Chen, Eric Ruike Zhao, Jingting Li, Ghanim Hableel, Jeanne E. Lemaster, Yuting Bai, George L. Sen, and Jesse V. Jokerst from UCSD and Gongyi Li from the National University of Defense Technology in China.
References:
- Chen, G., et al., Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chemical Reviews, 2016. 116(5): p. 2826-2885.
- Shi, J., et al., Cancer nanomedicine: progress, challenges and opportunities. Nature Reviews Cancer, 2016. 17: p. 20.
- Zhang, H.Y., et al., Processing Pathway Dependence of Amorphous Silica Nanoparticle Toxicity: Colloidal vs Pyrolytic. Journal of the American Chemical Society, 2012. 134(38): p. 15790-15804.
- Cacchioli, A., et al., Cytocompatibility and Cellular Internalization Mechanisms of SiC/SiO2 Nanowires. Nano Letters, 2014. 14(8): p. 4368-4375.








