
Boyle’s law, sometimes referred to as Mariotte’s law, is a mathematical law that describes the behavior of a sample of an ideal gas. Boyle’s law states that for a fixed amount of gas at a constant temperature, the volume of the gas is inversely proportional to the pressure of the gas. In other words, Boyle’s law tells us that as the volume of a gas decreases, the pressure of that gas increases. Mathematically, Boyle’s law can be expressed in two ways:
PV = k, or
P ∝ 1/V
Where P is pressure, V is volume, and k is a constant. This mathematical law tells us that the product of pressure and volume is constant for a given sample of gas, assuming the temperature remains constant. Since the product of pressure and volume is constant, the product of the pressure and volume in one state should equal the product of the pressure and volume in another state. Mathematically that is:
P1V1 = P2V2
This equation lets us take a sample of gas in one state and predict what the pressure or volume will be in another state, assuming the temperature remains constant.
Boyle’s law is one of the 4 gas laws, each which describe the behavior of a sample of an ideal gas. The other three laws, Charles’ law, Gay-Lussac’s law, and Avogadro’s law can be combined with Boyle’s law to give you the ideal gas law, an equation that describes the state of any hypothetical ideal gas.
Boyle’s Law
Boyle’s law states that the pressure and volume of an ideal gas at a constant temperature are inversely related. The derivation of this law relies on a few assumptions about the nature of the gas. An ideal gas is assumed to be made out of point particles that do not exert intermolecular forces on each other. Ideal gas particles are also assumed to have perfectly elastic collisions in which no energy is lost. In actually, gas molecules have a non-zero size, exert intermolecular forces on each other, and do not undergo perfectly elastic collisions. These discrepancies are normally too small to be significant for experimentation as most gases behave like ideal gases at moderate temperatures and pressures.
Mathematically, Boyle’s law is:
PV = k
In this equation, P is given in atmospheres (atm) and V is in liters (L). This law rests on the assumption that the temperature of the gas stays the same. As long as the temperature stays the same, the gas has a constant amount of energy, so k should stay that same.
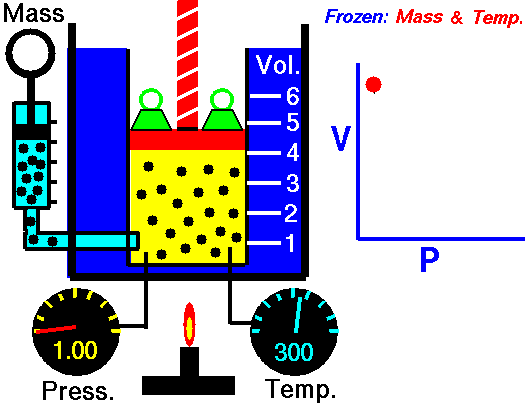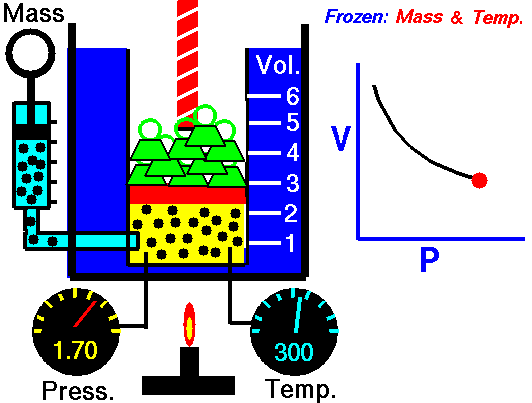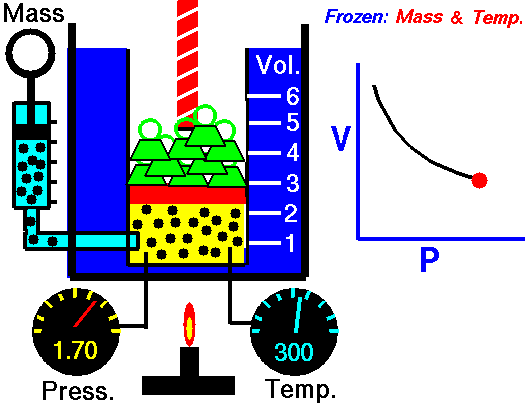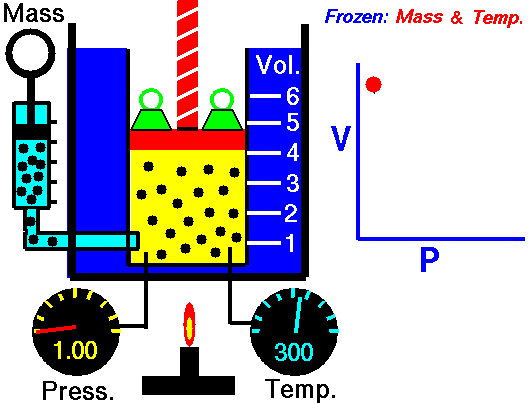
An animated image showing the relationship between pressure and volume. As volume decreases, pressure increases. Credit: WikiCommons CC0 1.0
Why should we expect this law to hold? Simply thinking about the nature of a gas should let us see the connection. Gases are made out of billions of tiny individual particles whizzing around randomly through space. As these particles move around, they bump into the walls of their container and produce a force, i.e. pressure. Now imagine we slowly shrink the container while keeping the amount of gas and the temperature the same. Temperature is a measure of the kinetic energy (movement) or particles, so keeping the temperature constant means that the particles have the same speed and momentum. Because the container is shrinking the molecules have less space to move around in. Because the gas has the same speed and momentum, they still exert the same amount of force, but that force is distributed over a smaller area. So, the gas exerts more force per unit area, which is an increase in pressure.
If the temperature also changed during this process, then the kinetic energy (movement) of the particles would also change and Boyle’s law would not hold.
Boyle’s law gives us a simple equation to predict the behavior of gases. Let’s see this equation in action with some simple problems.
Sample Problems
Questions
Q1.) A canister of CO2 has a volume of 5.6 L and k = 0.54. What is the pressure of the CO2?
Answer: This one is easy. Simply plugging these values into our equation gives us:
P(5.6L) = 0.54
P = 0.54/5.6
P = 0.01 atm
So the pressure of a 5.6 L canister of CO2 would be 0.01 atm.
Q2.) A balloon full of helium (He) has a pressure of 3.6 atm and k = 1.31. What is the volume of the balloon?
Answer: Once again, plugging these value into our equation gives us:
(3.6atm)V = 1.31
V = 1.31/3.6
V = 0.36 L
So the volume of the helium-filled balloon at 3.6 atm would be 0.36 L.
Q3.) A canister of hexane has a volume of 2.9 L and a pressure of 1.45 atm. The volume is then changed to 0.65L. What is the new corresponding pressure?
Answer: This problem requires us to use the second formulation of Boyle’s law. We know that for the same sample of gas, the product of the pressure and volume in one state is equal to the product of the pressure and volume in another state:
P1V1 = P2V2
We can use this equation to solve the problem. We know that the products of the pressure and volume should be equivalent in both cases. We can set up our equation, and solve for the missing value:
(2.9L)(1.45atm) = (0.65L)P
13.05 = (0.65)P
13.05/0.65 = P
P = 20.08 atm
The hexane would have a P = 20.08 atm
Q4.) 352 mL of chlorine under a pressure of 680 mm Hg are placed in a container under a pressure of 1210 mm Hg. What is the volume of the container in liters?
Answer: Once again we need to find a missing parameter of a gas. plugging in these values gives us our equation gives us:
(680 mm Hg)(352mL) = (1210 mm Hg)V
V = 198 mL
The same container of chlorine has a new volume of 198 mL.
Q5.) A company makes a ballon that is made to be inflated to a volume no greater than 3.0 L. If the balloon is filled with 2.3 L of helium at 1.67 atm, and rises in the atmosphere until the pressure is 0.71 atm, will the balloon burst?
Isolating our variables, we get V1 = 2.3, P1 = 1.67, and P2 = 0.71. Plugging these values into our equation gives us:
(2.3L)(1.67atm) = V(0.71atm)
V2 = 5.41 L
So yes, the balloon would burst.
Limitations Of Boyle’s Law
Since Boyle’s law rests on a few idealized assumptions about the nature of a gas, it is not a perfect description. Most gases behave according to Boyle’s law at moderate temperatures and pressures, or at least well enough for any practical purposes. At sufficient temperatures, any intermolecular attractions that could affect the motion of molecules are overcome by their kinetic energy. At very low temperatures where particles are moving slowly though, intermolecular forces become pronounced enough to have a significant effect on the behavior of the gas and Boyle’s law is no longer accurate.
Boyle’s Law And The Ideal Gas Law
Boyle’s law, along with Charles’ law, Gay-Lussac’s law, and Avagodro’s law can be combined to give a single expression called the ideal gas law. The ideal gas law is a state equation that represents the behavior of a gas under a number of different conditions. In a nutshell, the ideal gas law states that the pressure and volume of a gas are proportional to the temperature and amount of a gas. Mathematically, the ideal gas law can be written as:
PV = nRT
Where n is the amount of gas in moles, T is the temperature in Kelvin, and R is the universal gas constant. The ideal gas law allows us to predict how gas’ behavior will change in response to a change in one of its parameters.
History Of Boyle’s Law
Boyle’s law is named after the English/Irish chemist Robert Boyle, who is credited with the first experimental confirmation of the principle in the mid-17th century. Boyle and his assistant, another well-known scientist named Robert Hooke, invented a device that allowed them to directly control the pressure on a sample of gas to note any changes in volume. Boyle device involved an inverted J-tube, with a quantity of mercury forcing a gas into the closed portion. Boyle observed that the more mercury he put into the tube, the smaller the space the gas would occupy. Unlike his contemporaries who saw air as one of the 4 fundamental substances, Boyle believed that air was a semi-elastic fluid made of tiny particles. Boyle reasoned that the weight of the mercury itself compressed the air in the tube and figured that the more pressure, the more the air would be compressed.
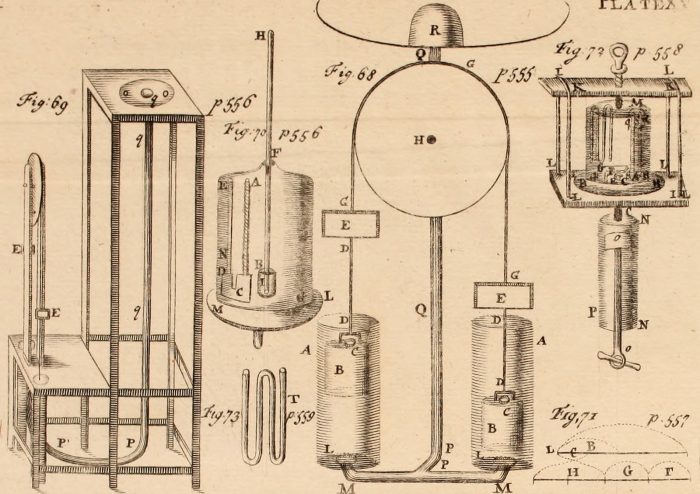
A drawing in one of Boyle’s manuscripts, outlining a device that allows an experimenter to control the pressure on a sample of gas. Credit: Internet Archive Book Images via Flickr Public Domain
Another French chemist named Edme Mariotte discovered the law independently around the same time, but Boyle had published it first. Mariotte did, however, show that the law only holds when the temperature of the gas is constant. Boyle’s law is considered the first scientific law expressed as a mathematical dependency between two variable quantities.
Boyle himself was also well-known for his advocacy for corpuscularianism—the view that matter is composed of smaller particles. Instead of describing the world and change in terms of Aristotelian, forms and substances and the 4 primary substances earth, wind, water, and fire, corpuscularians argued that reality and change could be accounted for by the motion of elementary particles. Boyle’s metaphysical views about the nature of chemical entities directly informed his experimentation, as he believed modern science could demonstrate his corpuscularian theory to be correct.








