Diffusion refers to the net movement of atoms or molecules from regions of high concentration to regions of low concentration. Molecules or atoms will move from regions of high concentration to regions of low concentration due to random particle movement, which results in a change in the concentration gradient of a sample. Diffusion is an important physical process and plays a role all over science from physics to cellular biology to sociology.
For example, if I release a sample of gas into a container, over time the molecules composing that gas will tend to spread out to evenly occupy the space in the container. Similarly, if I put a few drops of food dye in a sample of water, eventually the food dye molecules will spread out to be evenly distributed in the sample of water. Diffusion even happens in solid matter too, just normally at a rate too slow to be of any practical interest.
“The advancement and diffusion of knowledge is the only guardian of true liberty.” — James Madison
Although originally developed for chemistry, the concept of diffusion has found applications in other scientific fields. Mathematical diffusion models are used in biology to describe the motion of substances through cellular membranes, in economics to describe the flow of capital and prices in markets, and in sociology to describe the spread of ideas and values through society. In each case, some object (atoms, molecules, price, ideas, etc) starts out in a place of high concentration and “spreads out” to places of low concentration.
Why Does Diffusion Occur?
A characteristic feature of diffusion in chemistry is that it requires no net input of energy to occur. Molecules will spontaneously spread out to fill a space without being pushed around by any external force or input. Of course, the question arises, if diffusion requires no energy to occur, then what drives the process?
Consider the point this way, for any sample of gas in a space, there is a finite amount of ways that the particles in that gas could occupy that space. The overwhelming majority of these possible arrangements are ones where the gas is more evenly distributed in the space than not. Therefore, any random motion of molecules is more likely to put the system in a state that is closer to diffusion equilibrium simply because the vast majority of possible states the system could be in are ones where the particles in the gas are spread out evenly.
In simpler terms, diffusion is a statistical result of the random motion of particles. Nothing “causes” diffusion to occur in the sense of an external agent influencing a system. Given enough time, two substances will diffuse into each other simply because it is more likely to occur than not. Diffusion is a statistical truth about the possible physical arrangements of a system.
Important Processes That Involve Diffusion
Osmosis is an important biological process that occurs in cells that is the result of the selective diffusion of oxygen and water across a semi-permeable membrane. Biological cells are surrounded by a semi-permeable phospholipid membrane that regulates the flow of substances in and out of the cell. The membrane allows small molecules like oxygen, water, nitrogen or various lipids across and keeps out larger molecules like proteins and polysaccharides. Due to the presence of the selectively permeable membrane, substances will move from areas of a low concentration of solute to areas with a high concentration of solute. For example, if you submerge a human cell in salt water, the water molecules will move out of the cell to a region with a high concentration of salt. Conversely, if a cell is placed in fresh water, water molecules will move into the cell where there is a higher concentration of solvents.
“What I’m doing is obeying the law of diffusion of innovations.” — Simon Sinek
Osmosis is the result of the process of diffusion in the face of a selective membrane. The membrane controls the rate of diffusion by only allowing certain particles past, and so the result is a movement of a solvent across a low concentration gradient to a high concentration gradient. The process does not require any net energy input as the flow across the gradient is due to osmotic pressure on either side of the membrane. In biology, the movement of materials across a membrane absent the presence of external input of energy is called “passive transport.”
Diffusion is also crucial in the respiratory organs for providing oxygen to and removing carbon dioxide from the blood. Oxygen from inhaled air enters the alveoli of the lungs, tiny sacs composed of extremely thin and delicate membranes that allow the passive transport of oxygen and carbon dioxide. since the concentration of oxygen in the air is higher than that the blood, oxygen will diffuse across the membrane into the blood. Similarly, carbon dioxide diffuses from blood into the lungs to be exhaled out of the body. This delicate balancing act replenishes our blood with oxygen and expels carbon dioxide from the body
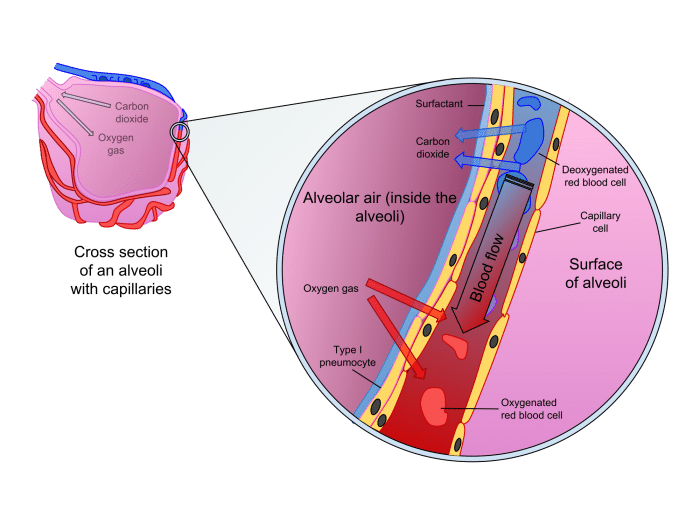
Diffusion in the alveoli. Exygen in the inhaled air diffues into the blood while carbon dioxide from the blood diffuses into the lungs to be expelled. Source: Delmalani18 via WikiCommons licensed under CC-BY-SA 4.0
Emphysema is a long-term degenerative condition of the lungs caused by the degradation of alveolar tissue. Damage to the alveolar membrane prevents the proper diffusion of oxygen and carbon dioxide in the lungs, resulting in shortness of breath and decreased blood oxygen content. Bronchitis is also a condition affecting the alveoli of the lungs, in which inflammation of alveolar tissue prevents oxygen diffusion.
“There is no cure for emphysema, but you can start treating it and have a better quality of life.” — Loni Anderson
In summation, diffusion refers to the tendency of molecules to spontaneously move from regions of high concentration to regions of low concentration. Diffusion is due to the random motion of particles and does not require a net energy input to occur. Diffusion is an extremely basic phenomenon and is involved in processes vital to life. If diffusion did not occur, the body would be unable to move a lot of its materials around and biological processes could not proceed.








