
A lipid is a biological molecule that dissolves (is soluble) in nonpolar solvents, and the monomers of lipids are fatty acids and glycerol. To better understand what this means, let’s take a look at both lipids and monomers in the context of organic molecules.
We’ll begin by seeing what the definitions of both monomers and lipids are.
What Are Monomers?
A monomer is a subunit or small portion of a larger organic molecule. You can think of this as small molecules that are chained together to create larger more complex molecules. Complex organic systems like the bodies of humans and other animals are comprised of monomers and there are different types of monomers including carbohydrates, lipids, proteins, nucleic acids.
Monomers have to be connected together properly, and the connection of monomers is done through the process of polymerization. Polymerization is the way two different molecules are connected together through the process of electron sharing, making a covalent bond between the two different molecules.
Polymers are simply made out of many repeating monomers, linked together to create a long chain. The amount of other molecules that a monomer can bond with is the result of how many active sites the molecule has, the number of places where covalent bonds can be created. Monomers that have polyfunctionality are those which can bond with at least two other monomers. The number of bonds that are present within the formed polymer affects the structure of the polymer. If the monomer is only capable of bonding with two other molecules than the polymer the results will have a chain format, meanwhile if the polymer can end up bonding with three molecules or more than it will have a 3D structure with molecules linked together at different angles.
The majority of monomers are organic in nature, although there are some synthetic monomers. Amino acids and nucleotides are examples of organic monomers with amino acids being those natural monomers that link together to create proteins and nucleotides are those which link together to form RNA and DNA.
Examples of synthetic monomers include ethylene gas which is used to create polyethylene. Another monomer used to create synthetic polymers is vinyl chloride, which plays a role in the creation of volume or polyvinylchloride or PVC, which itself is frequently used in the construction of plastic. Many bottles, building materials, and toys use PVC. Modified ethylene is also used in the creation of tetrachloroethylene which is an ingredient in the creation of Teflon.
What Are Lipids?
Lipids are organic compounds that naturally occur and are commonly known as oils and fats. One of the distinguishing characteristics of lipids is that they are not water-soluble. The primary function of lipids within the body is to store energy, send signals in between different components of the body, and make up the structure of the cell membrane.
Lipids are not water-soluble, they require at least one organic solvent to be soluble. The other major organic compounds like carbohydrates, nucleic acids, and proteins all dissolve much more easily in water than in solutions of organic solvent. While lipids are hydrocarbons, lipids don’t have a common molecular structure. Lipids which have ester function groups are different from other lipids in that they can be hydrolyzed in water. Neutral waxes, glycolipids, waxes, and phospholipids are all hydrolyzable. Lipids that don’t have the ester function group aren’t hydrolyzable, and they include vitamins A, E, D and K.
“My organs are too powerful… I manufacture blood and fat too rapidly.” — Robert Baldwin
As mentioned above, lipids are fats and oils and common lipids include things like vegetable oil, cholesterol, butter, fat-soluble vitamins, and waxes. What is true of most of these compounds is that while they are not water-soluble they will dissolve in a solution made out of one or more organic solvents.
Lipids play a variety of roles within organisms. Organisms mainly use lipids to store energy as well as for transferring messages between cells. Lipids are also used as a signaling molecule and to create the structure of a cell. Vitamins A, D, E, and K are fat-soluble vitamins and they are all lipids based on isoprene, which are stored in the body’s fat and the liver. The body can synthesize some kind of lipids, while other types of lipids must be obtained by consuming objects with those lipids in them. Lipids that the body cannot make and must be found in food include sterols, triglycerides, and membrane phospholipids or cholesterol. Lipogenesis is the process of producing lipids made out of carbohydrates gained through eating food.
While lipids don’t have a single common structure that defines them, the most commonly observed class of lipids are those known as triglycerides – oils and fats. Triglycerides are made out of a glycerol backbone and three fatty acids. If the acids which are bonded to the glycerol backbone are all the same, then the structure is referred to as a simple triglyceride. If the three fatty acids are different, then the triglyceride is dubbed a mixed triglyceride. Oils are triglycerides which have the distinction of being liquid at room temperature, and fats are those which are either semisolid or solid at room temperature. While oils are found more often in fish and plants, fats are more commonly found in other animals apart from fish.
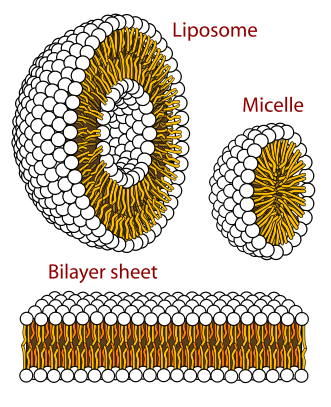
Phospholipids are used to create lipid bilayers and other structures. Photo: LadyofHats via Wikimedia Commons, Public Domain
While triglycerides are the most abundant class of lipids, the second most commonly occurring lipids are phospholipids. Phospholipids are found within the membranes of plant cells and in animal cells, and they contain the fatty acids and glycerol within them. Phosphoric acid is an ingredient of phospholipids. The most common types of phospholipids include cephalins and lecithins.

Prostacyclin is a type of fatty acid. Photo: Public Domain, https://commons.wikimedia.org/w/index.php?curid=1598315
Fatty acids that do not possess carbon-carbon double bonds are referred to as saturated fats, and these fats are usually solids and found in animals. Unsaturated fats are those which mainly come from plants, and they are fats that have two or more double bonds. Many unsaturated fats are liquid at room temperature due to the fact that the double bonds make it hard for multiple molecules to be packed together. In general, unsaturated fats have a lower boiling point than saturated fats do.
It is suspected one of the common causes of obesity is an excess of stored fats/lipids in the body. While some studies suggest there is a link between fat consumption and diabetes, many other studies have found that there is no explicit link between dietary fat and conditions like heart disease or cancer. Instead, weight gain is usually thought to be the product of overconsumption of any type of food, combined with individual metabolic factors.
“My diabetes is such a central part of my life… it did teach me discipline… it also taught me about moderation… I’ve trained myself to be super vigilant… because I feel better when I’m in control.” — Sonia Sotomayor
Other Types Of Monomers
Apart from lipids, other types of monomers include carbohydrates, proteins, and nucleic acids.
Carbohydrates
Carbohydrates are are long chains of monomers that are connected together, and they are responsible for storing the energy that is found in food. Glucose is a common monomer that has the formula C6H12O6. Glucose is primarily made by plants as they photosynthesize, and then animals eat the plants to gain this energy. Other simple sugars made out of carbohydrate monomers include fructose and galactose. These other simple sugars have the same chemical formula as glucose but they have different monomers – are structured differently. Animals store energy with a polysaccharide known as glycogen.
Proteins/Amino Acids
Proteins are made out of building blocks referred to as amino acids. Amino acids are made out of glucose molecules with carboxyl groups (COOH), an amine group (NH3) and an r-group side chain. There are 20 different amino acids that are combined together in different ways to create many different proteins, and these proteins are used by organisms to carry out a wide variety of different functions.
Proteins are joined together by peptide/covalent bonds, and there are different terms for proteins made up of different numbers of amino acids. Two amino acids linked together create a dipeptide, while three amino acids create a tripeptide. Naturally, four amino acids are joined together to make a tetrapeptide.
When amino acids are bonded together in chains this is called a primary structure, and creation of secondary structures or forms can occur when hydrogen bonds lead to the creation of beta-pleated sheets and alpha-helices. When amino acids are folded they create activated proteins and reside in a tertiary structure. Tertiary structure amino acids can be folded again to create complex yet stable quarternary structures like collagen.
Nucleotides

Photo: By OpenStax – https://openstax.org/books/anatomy-and-physiology/pages/preface, CC BY 4.0, https://commons.wikimedia.org/w/index.php?curid=30131206
Nucleotides are the blueprint or basis of construction for amino acids, storing the information needed to create them. Nucleotides are the monomers created from nucleic acids such as ribonucleic acid or RNA and deoxyribonucleic acid or DNA. RNA and DNA both hold an organism’s genetic code, and the nucleotide monomers are constructed out of a nitrogenous base, a phosphate, and a five-carbon sugar. The bases in DNA include cytosine, thymine, adenine, and guanine. Cytosine and thymine are both derived from purine, while the others are derived from pyrimidine. RNA does not have thymine; instead, it has a base called uracil which is also created from pyrimidine.









