Metal-organic frameworks (MOFs) are proven to be ideal platforms for heterogeneous catalysis and have been used in a wide variety of chemical reactions, showing promising catalytic performance. Due to the multicomponent nature of MOFs, active sites can be functionalized on metal nodes or organic struts, or even encapsulated within the pores.
Among these, linker functionalization provides a direct and efficient way to build MOFs with high loadings of catalytic-active sites. Numerous moieties with specific catalytic activities have been anchored on organic linkers to expand the scope of MOF-involved catalysis.
In our review work, Synthesis of MOFs for Heterogeneous Catalysis via Linker Design, we discussed recent advances in synthesizing MOF-based catalysts by using judiciously designed linkers in a catalog of different catalytic active sites (organometallic complexes, organocatalysts, BrØnsted acids). We pointed out the advantages of such linker design rationale: 1) protecting the catalytic active sites from deleterious agglomeration; 2) tailoring the chemical environment of catalytic sites with ease; and 3) introducing high loading and uniform distribution of catalytic sites to the MOF scaffolds. To take a deeper look, we also summarized the reactions catalyzed by those functionalized materials along with major findings on the relationship between MOF structures and their catalytic performance.
In the first part of our review, we summarized organometallic complexes that have been embedded on the organic building blocks of MOFs for catalysis. One the one hand, these organometallic complexes can be functionalized with coordinating groups, carboxylate for example, and directly be utilized as linkers to build MOF structure. On the other hand, they can be introduced through post-synthetic metalation, that is, organic linkers are firstly designed with open chelating metal sites to construct MOFs, then the functionalized MOFs complexes with desired metals to form catalytic sites.
Nowadays, various organometallic complexes like metalloporphyrins, polypryridyl metal complexes, N-heterocyclic carbene (NHC) complexes, pincer complexes, etc. have been incorporated into MOFs and these organometallic complexes enabled the MOFs to catalyze many classic organic transformations including cycloaddition of CO2, cyanosilylation, hydrogen evolution, reduction of CO2, and so on.
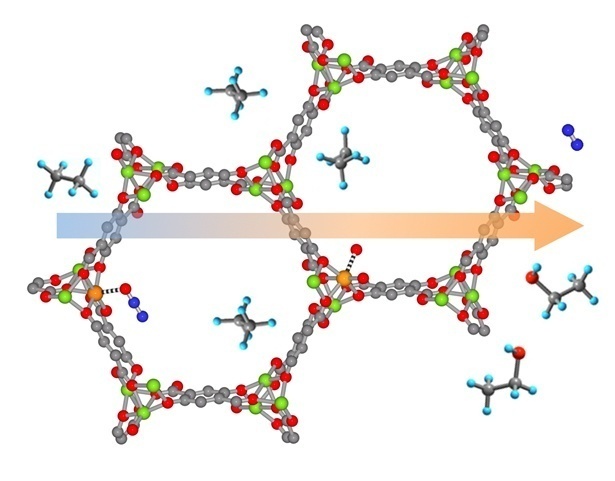
Secondly, we talked about organocatalytic moieties that have also been introduced into the MOF struts via judiciously linker design. In one case, Hydrogen-bond-donating (HBD) organocatalysts bearing urea, thiourea and squaramide moieties have been designed as organic linkers of MOFs. With the N-H bond, these linkers can provide two-point hydrogen bonding, ultimately activating substrates for the subsequent addition reactions. In another case, chiral moieties such as proline and peptides were used to build chiral MOFs in the sake of catalyzing enantioselective reactions like asymmetric aldol addition.
Last but not least, we discussed the MOFs functionalized with BrØnsted acids, especially sulfonic acid. A serial of sulfoxy-acid-functionalized frameworks has been prepared in the pursuit of catalyzing acid-driven reactions, such as esterification condensation of diamines, methanolysis, acetalization, isomerization, and cycloaddition. Similar to the previous motifs, BrØnsted acids can be preinstalled on the organic linkers before the MOF synthesis or anchored on the backbones via post-synthetic modification of the original MOFs.
Due to the fast development of MOF-based heterogeneous catalysis, we will keep a track on the field, exploring the utilization of the materials in the real-world applications.
These findings are described in the article entitled Synthesis of MOFs for Heterogeneous Catalysis via Linker Design, recently published in the journal Polyhedron. This work was conducted by Yingmu Zhang, Xinyu Yang, and Hong-Cai Zhou from Texas A&M University.








