
Different cancer treatments exist, but they each have variable efficacies and non-negligible side effects
Many innovative approaches are under development in research labs or are being tested in phase I/II trials. Among such treatments are genetically-engineered CAR T cells that bypass the natural function of patients’ T cells to efficiently target and kill tumors. Two CAR T cell products were recently approved by the FDA, and their administration is limited to certified centers in the US.
Dedicated manufacturing setups are necessary to produce such cells, rendering them more expensive than conventional treatments and less accessible to patients. CAR-T cells will possibly herald the first commercial success of industry-manufactured cellular therapies, an activity that has historically been developed and supported by academia in an organizational context different than the industrial pharma framework.
In this review, we discuss the hurdles on the path to commercialization of CAR T cells. The complexity of the supply chain, from primary cell collection to administration of a personalized medicinal product, is hardly amenable to the conventional central manufacturing processes used for conventional drug batch production. This warrants exploring whether alternative models of manufacturing (centralized versus point-of-care) is compatible with modern regulations, patient safety, and cost containment.
In part — but not only — because current organizations for manufacturing necessitate cross-border shipping of the starting material and of the medicinal product ready to be administered, a harmonization of regulations is needed to speed up access for patients in many countries. To paraphrase one of the first patients treated with CAR-T cells in the US, a treatment that is not accessible to many is not a cure at all.
CAR T cells also carry significant risks of toxicities. For broad applicability, engineering safety mechanisms into the gene constructs of CAR T cells is required.
We also discuss avenues to increase the likelihood of commercial success of CAR T cells:
- Point-of-care technologies can reduce the complexity of the supply chain by collecting cells from a patient, manufacturing CAR T cells, and infusing to the same patient at the same site;
- Adopting an off-the-shelf approach for readily-available stocks/batches of CAR T cells accessible to large numbers of patients, as opposed to the current model of patient-specific CAR T cells; and
- Building on the success of CAR T cells in leukemias and lymphomas to target other more prevalent tumors, such as solid tumors, is a most desirable goal, however, this implies a better knowledge of tumor antigens, trafficking of immune cells in the vicinity of tumor cells, and the immunosuppressive properties of the tumor microenvironment.
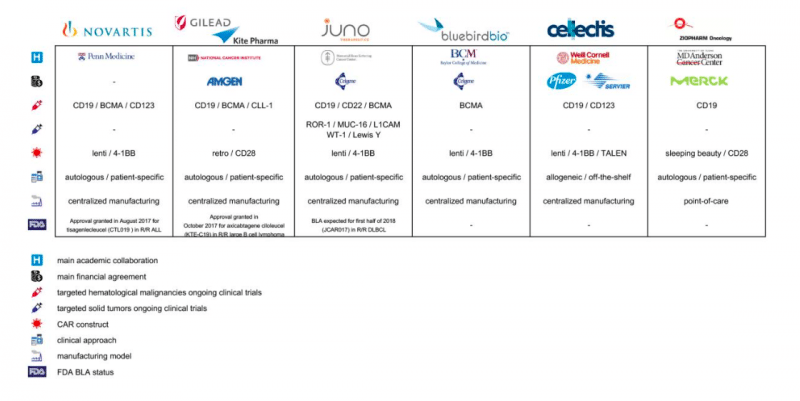
Figure: Major pharmaceutical industry players currently contributing to developments in the CAR-T cell field (Republished with permission from Elsevier)
These findings are described in the article entitled, From clinical proof-of-concept to commercialization of CAR T cells, recently published in the journal Drug Discovery Today. This work was conducted by Boris Calmels, Bechara Mfarrej, and Christian Chabannon from Aix Marseille University and the Institut Paoli-Calmettes.








