
Medicine, like other areas of human activity, is full of paradoxes. Unfortunately, paradoxes in medicine are often associated with wrenching cost – human lives.
While solving a medical paradox is usually a noticeable triumphal move, there is one subtle crucial step before, without which nothing could happen. It is the recognition of a paradox. Someone could think it should not be difficult to recognize an event that contradicts common sense, whatever the paradox is. However, history of medicine tells different stories.
One of the most famous episodes of solving paradoxes happened in Austria in the middle of the 19th century.
There were the two almost identical obstetric clinics in Vienna. However, it “was the remarkable difference in puerperal fever mortality between the two neighboring clinics. In 1846, for instance, in the 1st Obstetric Clinic, where he worked, out of 4010 labouring patients, 459 had died of puerperal fever, a total of 11·4 per cent, while during the same period, in the 2nd Obstetric Clinic, out of 3 754 labouring patients only 105 had died, i.e. 2·7 per cent. Over a stretch of five years (1841- 1846), the 1st clinic had witnessed 1 300 more victims of puerperal fever than the 2nd clinic. … All the doctors of the hospital knew about this; and so did the laboring patients who were mortally scared of being admitted to Klein’s clinic (1st Obstetric Clinic – VS). However, these figures were only remarkable in the eyes of Semmelweis”[1].
A Hungarian physician, Ignaz Philipp Semmelweis, was the only one who recognized the paradox, and for years relentlessly searched for an explanation. Dr. Semmelweis found that the only difference between clinics was that the same medical personnel taking care of laboring women also routinely performed postmortem examinations of deceased patients, a practice in the first obstetric clinic but not in the second. Doctors and assistants barely washed their hands between these activities. The paradox became a logical event: the doctors’ hands carried the “cadaverous matter” to laboring women, and they had to be properly cleaned before examinations. The concept of Antiseptic was created, twenty years prior to the Louis Pasteur discovery.
Recognizing a paradox, i.e. to be aware of disagreement between anticipated and reality, is a natural function of human cognition, but to exercise or not is our choice.
All science is about causation. When we observe an event, if it is not consistent with our explanatory models, we ask why. In order to ask a question, we must see a discrepancy between what is observed and what the model predicts; the observation should be surprising. When I started a study of primary liver cancer or hepatocellular carcinoma (HCC), I was surprised by the unusual frequency of portal veins’ metastases [2].
In general, cancer is characterized by metastatic spread to distant organs, which accounts for 90% of cancer-associated deaths [3]. HCC is the third leading cause of cancer-related mortality and is on the rise worldwide. However, unlike other carcinomas, HCC metastasizes to distant organs only in minority of patients, whereas metastases to the liver and liver vasculature occur virtually in all HCC patients [4,5]. HCC cells disseminate systemically via hepatic veins, but portal veins are paradoxically affected by metastasis more frequently than are hepatic veins, which correlates with poor prognosis. This portal metastatic prevalence also occurs when the primary tumor is small [6,7] and in non-cirrhotic livers [8-12], or even when the primary tumor is not detected at all [13-20]. The phenomenon of preferential portal metastases is unique to HCC; other GI carcinomas always spread metastatic cells via the portal vein to the liver, but rarely colonize the portal veins themselves:
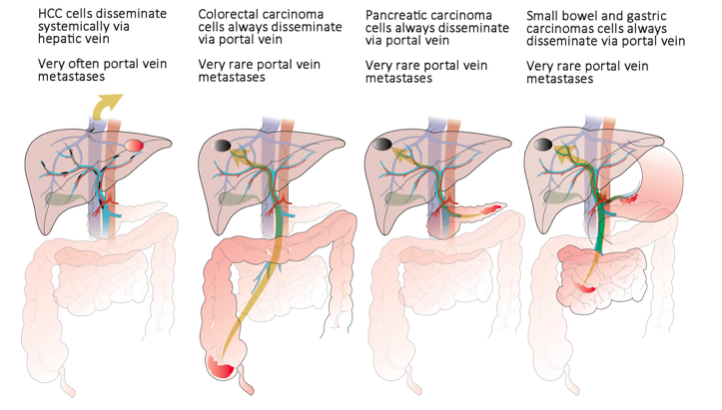
Republished with permission from Elsevier from: https://doi.org/10.1016/j.drudis.2018.01.020
So, are we surprised by this contradictional privileged HCC metastasis to portal vein? In general, not at all! There is only one publication that asked this question, it was contributed by Byung Ihn Choi in 1995, a prominent radiologist from Seoul National University. In his editorial, Dr. Choi straightforwardly asked, “What accounts for such discrepancy in the involvement of the portal vein and hepatic vein?” and outlined potential explanations, but none of them fully explained the paradox and neither offered the solution. After Dr. Choi’s unanswered quest, twenty-three years have passed by the paradox, and we never ask the question, “Why?” My guess is that we got used to it.
However, portal HCC paradox becomes a logical event in light of the Stephen Paget’s “seed and soil” hypothesis [21]. I propose that privileged HCC portal/liver metastasis is due to the presence of pancreatic family hormones and growth factors (PHGFs) in the portal blood, which constitutes the “soil,” while circulating HCC cells became “seeds” after exposure to the portal blood. Liver hepatocytes extract/consume the same PHGFs, creating less favorable conditions for HCC metastasis in hepatic veins and distant organs. I suggest that the portal vein environment, enriched with PHGFs, facilitates the selection of more aggressive HCC clones from portal metastasis, explaining the worse outcome with portal pattern present.
Based on facts that a unique “portal system” vascular design of “ancestral” chordate Amphioxus allows insulin-producing cells of the diverticulum to sense the glucose level in “portal” blood [22,23], the analysis hypothesizes that during early chordate evolution the acquirement of the portal system, carrying PHGFs back to enterocytes, promoted the differentiation of the enterocytes into hepatocytes, leading to the formation of the vertebrate liver.
These findings are described in the article entitled Privileged portal metastasis of hepatocellular carcinoma in light of the coevolution of a visceral portal system and liver in the chordate lineage: a search for therapeutic targets, recently published in the journal Drug Discovery Today. This work was conducted by Vladimir M. Subbotin from the University of Wisconsin and the University of Pittsburgh.
*This analysis was recently presented at The 25th The Asian Pacific Association for the Study of the Liver (APASL) Conference on “Hepatocellular carcinoma: Strategy in the New Era”, May 11-13, 2018, Yokohama, Japan.
References:
- Gortvay, G. and Zoltán, I.G. (1968) Semmelweis: his life and work, Akadémiai Kiadó.
- Marsh, J.W. et al. (1997) The prediction of risk of recurrence and time to recurrence of hepatocellular carcinoma after orthotopic liver transplantation: a pilot study. Hepatology 26 (2), 444-450 DOI: 10.1002/hep.510260227 (http://www.ncbi.nlm.nih.gov/pubmed/9252157)
- Labelle, M. and Hynes, R.O. (2012) The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov 2 (12), 1091-1099 DOI: 10.1158/2159-8290.CD-12-0329 (http://www.ncbi.nlm.nih.gov/pubmed/23166151)
- Carr, B.I. and Guerra, V. (2016) Hepatocellular Carcinoma Extrahepatic Metastasis in Relation to Tumor Size and Alkaline Phosphatase Levels. Oncology 90 (3), 136-142 DOI: 10.1159/000443480 (<Go to ISI>://WOS:000372522200003)
- Senthilnathan, S. et al. (2012) Extrahepatic metastases occur in a minority of hepatocellular carcinoma patients treated with locoregional therapies: analyzing patterns of progression in 285 patients. Hepatology 55 (5), 1432-1442 DOI: 10.1002/hep.24812 (http://www.ncbi.nlm.nih.gov/pubmed/22109811)
- Ikai, I. et al. (2005) Report of the 16th follow-up survey of primary liver cancer. Hepatol Res 32 (3), 163-172 DOI: 10.1016/j.hepres.2005.04.005 (http://www.ncbi.nlm.nih.gov/pubmed/16024288)
- Kamiyama, T. et al. (2007) Efficacy of preoperative radiotherapy to portal vein tumor thrombus in the main trunk or first branch in patients with hepatocellular carcinoma. Int J Clin Oncol 12 (5), 363-368 DOI: 10.1007/s10147-007-0701-y (http://www.ncbi.nlm.nih.gov/pubmed/17929118)
- Trevisani, F. et al. (2010) Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Digestive and Liver Disease 42 (5), 341-347
- Edeline, J. et al. (2009) Systemic chemotherapy for hepatocellular carcinoma in non-cirrhotic liver: a retrospective study. World journal of gastroenterology: WJG 15 (6), 713
- Grazi, G. et al. (2003) Liver resection for hepatocellular carcinoma in cirrhotics and noncirrhotics. Evaluation of clinicopathologic features and comparison of risk factors for long‐term survival and tumour recurrence in a single centre. Alimentary pharmacology & therapeutics 17 (s2), 119-129
- Shimada, M. et al. (2000) The importance of hepatic resection for hepatocellular carcinoma originating from nonfibrotic liver. J Am Coll Surg 191 (5), 531-537
- Nzeako, U.C. et al. (1996) Hepatocellular carcinoma in cirrhotic and noncirrhotic livers: a clinico-histopathologic study of 804 North American patients. Am J Clin Pathol 105 (1), 65-75
- Lim, J.H. and Auh, Y.H. (1992) Hepatocellular-carcinoma presenting only as portal venous tumor thrombosis – CT demonstration. Journal of Computer Assisted Tomography 16 (1), 103-106 DOI: 10.1097/00004728-199201000-00019 (<Go to ISI>://WOS:A1992GZ68700019)
- Kim, J.W. et al. (2013) Rapidly Progressive Hepatocellular Carcinoma Mimicking Benign Portal Vein Thrombosis: A Case Report. Gut Liver 7 (1), 116
- Yamashita, Y. et al. (2015) Predictors of Microvascular Invasion in Hepatocellular Carcinoma. Dig Dis 33 (5), 655-660 DOI: 10.1159/000438475 (http://www.ncbi.nlm.nih.gov/pubmed/26398341)
- Kim, J.W. et al. (2013) Rapidly progressive hepatocellular carcinoma mimicking benign portal vein thrombosis: a case report. Gut Liver 7 (1), 116-119 DOI: 10.5009/gnl.2013.7.1.116 (http://www.ncbi.nlm.nih.gov/pubmed/23423240)
- Mutai, J. et al. (2016) Isolated Portal Venous Hepatocellular Carcinoma. Journal of gastrointestinal cancer, 1-3
- Schaeffer, C. et al. (2000) Cytokine gene expression during postnatal small intestinal development: regulation by glucocorticoids. Gut 47 (2), 192-198 DOI: 10.1136/gut.47.2.192 (<Go to ISI>://WOS:000088380700010)
- Vilana, R. et al. (1993) Fine-needle aspiration biopsy of portal vein thrombus: value in detecting malignant thrombosis. AJR. American journal of roentgenology 160 (6), 1285-1287
- Rammohan, A. et al. (2013) Percutaneous ultrasound-guided fine-needle aspiration of portal vein thrombi as a diagnostic and staging technique for hepatocellular carcinoma. Abdominal imaging 38 (5), 1057-1060
- Paget, S. (1889) The distribution of secondary growths in cancer of the breast. The Lancet 133 (3421), 571-573
- Subbotin, V.M. (2017) Arguments on the origin of the vertebrate liver and the Amphioxus hepatic diverticulum: A hypothesis on evolutionary novelties. Vavilov Journal of Genetics and Breeding, Letters to the Journal 21 (4), 1-15 (http://www.bionet.nsc.ru/vogis/download/hypothesis/appx1.pdf)
- Subbotin, V.M. (2018) Privileged portal metastasis of hepatocellular carcinoma in light of the coevolution of a visceral portal system and liver in the chordate lineage: a search for therapeutic targets. Drug Discov Today 23 (3), 548-564 DOI: 10.1016/j.drudis.2018.01.020 (http://www.ncbi.nlm.nih.gov/pubmed/29330122)









