
Rational design and synthesis of appropriate, eco-friendly, and cost-effective catalysts with preserved chemical and energy functionalities is a vital research gap in the production of renewable and sustainable energy on an industrial scale. The production scheme for renewable and sustainable energy includes CO/CO2 hydrogenation, water-gas shift (WGS) reactions, hydrocarbon isomerization, and methane reforming, and the commercially available catalysts for the processes are group 9 and 10 noble metals (Pt, Pd, Rh). These catalysts are limited since they are cost-intensive.
Recently, Molybdenum Carbide nanoparticles (MCN) have been utilized as a replacement for Ru, and Pt as electrocatalysts for the anode of polymer membrane fuel cells (PEMFC) due to its platinum-like properties. MCNs exhibit unique physical and chemical properties, which enhanced their popularity in the fields of materials and chemical science towards the production of renewable and sustainable energy. MCNs are thermally stable and possess high adsorption capacity, electrical conductivity, hardness, and melting point.
Moreover, MCNs are resistant to sulfur and nitrogen, and their durability and catalytic current density are like those of the noble metals. These make MCNs suitable hydrogen evolution reactions (HER) and hydrogenation reaction such as hydrodeoxygenation (HDO) reactions, hydrogenation of CO to form alcohol, hydrogenation of CO2 to form alcohol, electrocatalytic hydrogen evolution from water splitting including hydrodenitrogenation (HDN), WGS, CH4 aromatization, hydrotreating, oxygen evolution reaction, and hydrodesulfurization (HDS).
The structure of MCN is complex, with several stable and metastable phases, making it difficult to understand its structural diversity and suitability for industrial application. However, the crystal structure of MCNs can be identified using density functional theory (DFT) based computation with revised Perdew–Burke–Ernzerhof (RPBE) exchange-correlation function. This shows that MCN has five different crystal structures, including α-Mo2C, α-MoC1−x, β-Mo2C, η-MoC, and γ-MoC, depending on the preparation procedure and the carburizing agent used.
Five different crystal structures of Molybdenum carbide nanoparticles
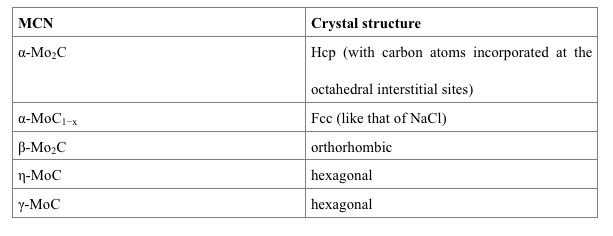
The computational study of the crystal structure shows that β-Mo2C and α-MoC1−x are more stable and exhibit amazing electrochemical activity because of their large ionic contribution. β-Mo2C is more efficient for Water-Gas Shift (WGS) reaction, substituting the Cu-based commercial catalysts, while α-Mo2C is efficient for dehydrogenation reactions. Their structural functionalities largely depend on their surface properties.
The reaction mechanism of MCN can be explained using multiscale reaction model (QM/MM), by combining molecular mechanical (MM) force field with density functional tight-binding (DFTB) technique and quantum mechanical (QM). Nevertheless, the computational cost is expensive due to the required accuracy. In the quest to lower the computation cost of the model without penalizing the accuracy, artificial intelligence technique, as well as machine learning has been proposed.
Neural-network (NN) is a viable artificial intelligence technique good for prediction of the ab initio potential energy surface (PES). NN PES can produce the same result with that of DFT at a minimal computational cost. However, the use of extreme learning machine (ELM) developed from NN is a more promising technique because ELM is extremely faster in learning than NN, making it more suitable for high-dimensional potentials, and possess better generalization capability and efficiency.
To enhance the catalytic performance of MCN, it can be supported/promoted to form an MCN-based nanocomposite. This will help to boost conversion, yield, turn over frequency (TOF), selectivity, as well as product distribution. For instance, MCN with platinum support (Pt/Mo2C) is suitable for WGS reaction, having the capacity to offer a remarkable WGS rate (mol CO/molPt s) superior to the rates reported for commercial Cu-Zn-Al catalyst and active oxide supported Pt (like Pt/TiO2, Pt/CeOx, and Pt/CeO2). MCN with TiO2 support (Mo2C/TiO2) is a suitable alternative catalyst for HDO of fast pyrolysis of phenol (a bio-oil model compound), with a high selectivity for benzene and moderate catalyst deactivation.
The performance of MCN can also be enhanced by using additives like La Mg, Co, Ce and K, and supporting with Ni by impregnation (especially for tri-reformation of methane). For instance, the addition of La to Ni supported MCN helps to prevent aggregation of nanoparticles to facilitate a topotactic transformation of MCN species and suppressed deposition of carbon, resulting in outstanding catalytic performance. However, the addition of K and Mg to Ni supported MCN, particularly the former, displayed a dramatic reduction in the redox activity in methane tri-reforming.
The catalytic activity of MCN can also be enhanced by using carbon support such as activated charcoal, carbon black, carbon nanotubes (CNTs), and graphitic mesoporous carbon (GMC). They have been efficiently used for catalytic hydrogenation of CO to form mixed alcohol. For instance, the use of GMC helps in forming smaller carbide particles, resulting in improved catalytic performance. Furthermore, the rational addition of K2CO3 to β-Mo2C/GMC (0.05–0.5 K/Mo molar ratio) can significantly promote the production of C2+–OH, leading to a maximum space-time yield (STY) of C2+–OH at medium K/Mo ratio of 0.1. Addition of K2CO3 to β-Mo2C/GMC offers better STY and C2+–OH selectivity in comparison with a typical Rh/GMC catalyst promoted with oxide of Mn, Li, and Fe. CNT/MCN composite promoted with K favors formation of alcohol by suppressing formation of hydrocarbon. Further addition of Co to the nanocomposite could enhance CO conversion appreciably due to the formation of “KMo-C” and “Co3Mo3C” phases, which are responsible for the active formation of alcohols.
Therefore, the choice of supporter helps to saves time, possesses a better rate when compared with commercial Cu-Zn-Al catalyst and active oxide supported catalysts. Although the addition of supports could mean an extra cost of material and process, which is justifiable since supported MCN catalysts are more effective and function at a superior rate, thereby saving time and lowering the cost of using the catalyst for production of sustainable energy.
These findings are described in the article entitled Molybdenum carbide nanoparticle: Understanding the surface properties and reaction mechanism for energy production towards a sustainable future, recently published in the journal, Renewable and Sustainable Energy Reviews. The article was authored by Peter Adeniyi Alaba from Covenant University, together with Ali Abbas and Jun Huang from the University of Sydney, and Wan Mohd Ashri Wan Daud from the University of Malaya.








