
The discovery of nanostructured carbon material “fullerenes” has emerged as a new substitute for widely-accepted nanocarbon material like graphene in the world of nano-research. The advent of this new nanomaterial by Kroto et al. in 1985 gave it a chance to occupy major space in various applications like sensors, photovoltaics, catalysis, electronics, and so on. When laser vaporization of graphite results in the formation of 60 carbon clusters, they are termed “Buckminster fullerene,” or simply, “bucky ball.”
The uniqueness of C60 fullerene relies on its molecular organization, which is a truncated icosahedron with 12 pentagon and 20 hexagon ring structures (1). Extreme stability as a result of symmetrically-arranged carbon atoms in fullerenes makes them suitable for biomedical applications as a drug delivery agent, bioimaging probe, tissue engineering scaffolds, etc. Buckminster fullerene, or C60 fullerenes, are the most abundant and widely-used among the fullerene family. The fullerene group possesses a similar structure but with different number of carbon atoms. The major area in which fullerene receives attention in biomedical applications is the antioxidant potential that they exhibit in a biological system.
Oxidation reduction reactions as a part of mitochondrial respiration constitute the principal reactions where free radicals extensively generate and propagate inside the cell. Molecules that scavenge these highly reactive oxygen species can improve the survival rate of cells. Fullerenes with specifically arranged carbon atoms are good enough to uncouple the electron transport chain reactions that propagate inside the mitochondrial compartment. Characteristic 60 carbon arrangements also devote antimicrobial property to fullerenes via disruption of protective lipid bilayer and membrane structures of micro-organisms. Likewise, the doping of metal ions inside the bucky ball structure of fullerene is used as a contrast agent. In bioimaging, photoirradiation of fullerene-conjugates with DNA binding ability is often used as an anticancer agent (2) (Figure 1).
While considering these advantages, it is important to point out the major disadvantages of fullerene C60, like the hydrophobic nature that restricts their applicability in a biological system. Researchers overcome this problem via functionalization of fullerenes with various hydrophilic moieties on their surface (3). As a result, water-soluble fullerenes were established as a theranostic agent in osteoporosis and targeted drug/gene delivery agents in cancer treatment.
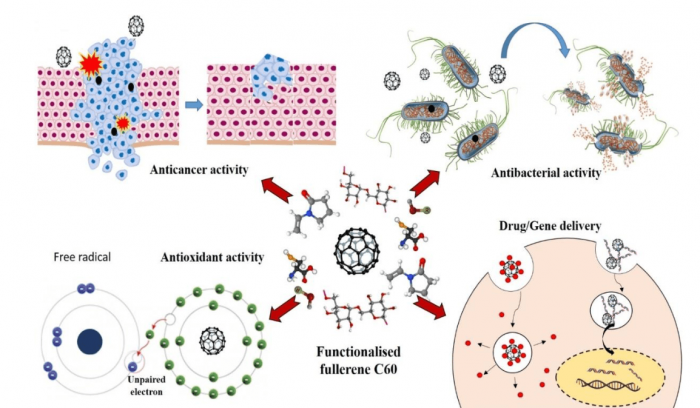
Figure 1: Biomedical application of fullerene C60. Figure courtesy Mohanan PV.
Advancement of nanotechnology research led to the development of materials with tremendous biomedical applications. However, the use of these materials as pharmaceuticals requires thorough investigation regarding their toxicity to living things and the environment. Although many studies reported the general toxicity of nanoparticles in living systems, controversies still exist about the mechanism and role of physico-chemical characteristics. The way by which most of the nanosized materials induce toxicity to a living system is through their direct interaction with the plasma membrane and associated destruction of membrane-protective units.
In another concept, if the particles are internalized into the cells via the endocytosis mechanism, they will be distributed in cytoplasm and access interaction with the cellular organelles. Some of the fine-tuned nanoparticles can cross the nuclear envelope and even induce damage to genetic materials (4). Hence, it is necessary to draw attention to the toxicological evaluation of synthesized materials and to prove their safety before using them for medical applications. The present study addresses the interaction of dextran functionalised fullerene C60 (Dex-F60) with Chinese Hamster Ovary cell lines (CHO).
The study begins with the functionalization of fullerene C60 using hydrophilic polymer: dextran and characterization of modified material using TEM imaging and FTIR analysis. Afterward, detailed toxicity profiling of Dex-F60 using CHO cells were conducted. Cellular viability in terms of mitochondrial reduction potential and lysosomal internalisation was assessed after 24h of Dex-F60 exposure with CHO cells. Generally, nanocompounds generate oxidative stress in cells as they distract the mitochondrial membrane potential and accelerate the evolution of free radicals. The present study reported increased generation of free radicals in cell cytoplasm after Dex-F60 interaction. Free-radical generation was found to depend on the concentration of Dex-F60 particles exposed to CHO cells. However, the cytoskeletal integrity as well as organelle function were not affected by Dex-F60 at the particular concentrations used for this study. The potential of Dex-F60 to impart any serious effect to nuclear counterparts was also investigated using DAPI staining and a DNA ladder assay. Flow cytometry was also conducted to check the number of live/dead cells present in the system after Dex-F60 interaction. There was no nuclear disintegration and apoptotic cell death found in the presence of Dex-F60. Major observations of the study are depicted in Figure 2.
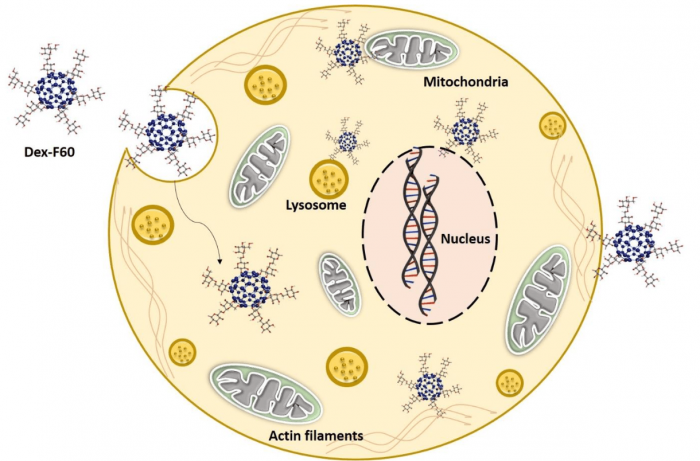
Figure 2: Biocompatibility of Dextran functionalized fullerene C60. Figure courtesy Mohanan PV.
The entire study concluded with a notion that dextran-stabilized Fullerene C60 is a biocompatible material that can definitely gleam in the field of nano-medicine.









