
With the development of nuclear power plant and industry, “radionuclide contamination” has increasingly seen worldwide attention. The study on the properties of radionuclides, especially in the natural environment can reveal more about the toxicity of radionuclides in the environment, thereby allowing people to deal with them more effectively.
The chemical species and microstructures of radionuclides at solid particle surfaces are closely associated with its transport, hazardous and bioavailability in the environment. Recently, professor Xiangke Wang from North China Electric Power University (China) reported a series of works on the interaction mechanism of radionuclides with different clay materials at a molecular level by the advanced synchrotron radiation technique and computational theoretical calculations.
Wang’s group applied X-ray absorption fine structure (XAFS) spectroscopy including X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) spectroscopy to provide the structure information (e.g., the bond distances and coordination numbers of neighbors) of radionuclides at a molecular level. They also applied plasma- and chemical-grafted amidoxime/carbon nanofiber hybrids (p-AO/CNFs and c-AO/CNFs) to remove 238U(VI) from wastewater. The chemical species and microstructures of U(VI) at the two kinds of nanomaterials are investigated by EXAFS (Environmental Science & Technology, 2017, 51, 12274-12282). Figure 1A and 1B showed the spectroscopy information of U(VI) on the nanomaterials, these characterizations suggested that U(VI) formed strong inner-sphere surface complexes even at low pH, which is very important for the application of such nanomaterials in the removal of U(VI) from wastewater.
The removal of U(VI) onto sericite in the presence of Bacillus subtilis (B. subtilis) is also studied using EXAFS techniques (Geochimica et Cosmochimica Acta, 2016, 180: 51-65). Figure 1C and 1D also show that inner-sphere surface complexation between U(VI) and sericite + B. subtilis at pH 7.0 and pH 4.0 are ascribed to the U-Al/Si or U-C shell and U-P shell, respectively.
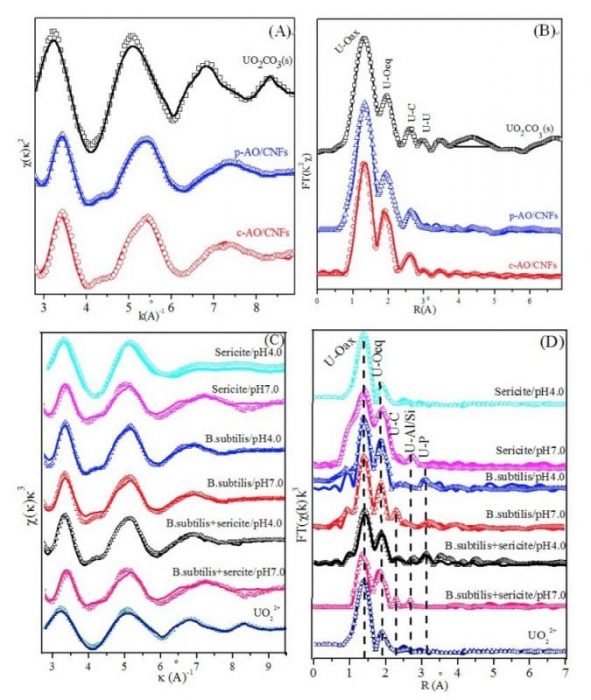
Figure 1. The k2-weighted U LIII-edge EXAFS spectra (A) and corresponding Fourier Transmission (FT) (B) of references and U(VI) sorption samples, CU(VI) = 10 mg/L, m/v = 0.6 g/L, I = 0.01 mol/L NaCl, T = 293 K. Uranium LIII-edge EXAFS spectra (C) and corresponding Fourier transform (D) for U(VI)-reacted sericite, B. subtilis and sericite + B. subtilis at pH 4.0 and 7.0 under atmospheric conditions, CU(VI) = 6.3 μmol/L, Csericite = 2.0 g/L, CB. subtilis = 0.5 g/L, I = 0.01 mol/L NaClO4, T = 293 K. Reproduced with permission from the publisher.
Due to the limited sensitivity of the XAFS technique, it is still challenging to use it on radionuclide analysis at environmental concentrations. Furthermore, for some actinides (e.g., Am, Np, Cm, Pu), the radionuclide operation license makes it difficult to carry out the XAFS measurements. Density functional theory (DFT), one of the most powerful theoretical tools, is applied efficiently to calculate correlation energy and electronic structures. For instance, the theoretical calculation provides the differences in the interactions between CNTs with Eu(III) and 243Am(III) ions (Environmental Science & Technology, 2015, 49, 11721-11728). Figure 2 shows that the charge transfer interaction between CNTs and Eu(III) are much stronger than that of 243Am(III). This can help us to understand the difference between lanthanides and actinides on nanomaterials.
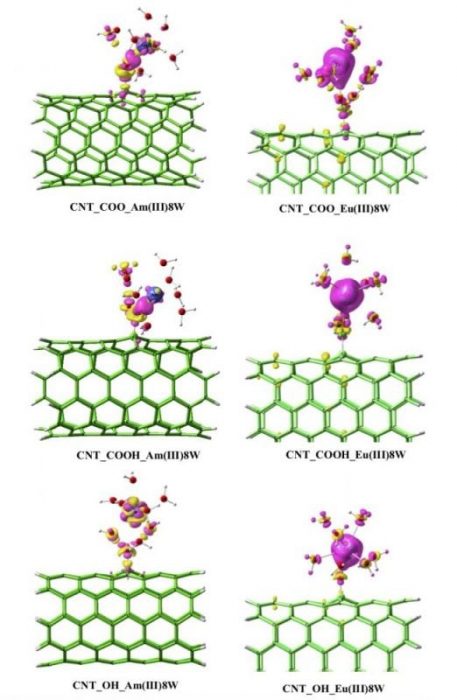
Figure 2. The difference density between the oxidized CNTs_M(III)8W and its two fragments (oxidized CNTs and M(III)8W). The violet color and yellow color represent the increase and decrease of electron density, respectively. Both of the two colors indicated that the charge transfer interaction exists. The significant increase electron density indicates the strong charge transfer interaction. Reproduced with permission from the publisher.
In order to more fully understand the transport of radionuclides in the natural environment, the combination of batch technique, theoretical calculation, and spectroscopy analysis are helpful to understand the interaction mechanisms and to safely predict the disposal of long-lived radionuclides.
The interaction between U(VI) and graphene oxides (GOs) with different surface properties are studied by the combination of EXAFS technique and DFT calculations (Environmental Science & Technology, 2015, 49, 4255-4262). Based on the EXAFS analysis (Figure 3A), the sorption of U(VI) on GOs with different surface functional groups are different, which is also evidenced by the theoretical calculations (Figure 3B).
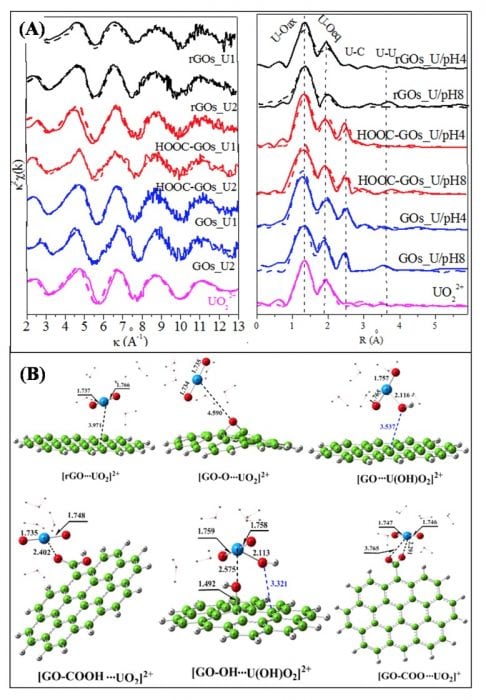
Figure 3. (A) The k2-weighted U LIII-edge EXAFS spectra (left) and the corresponding Fourier Transforms (right) of the reference and samples before and after desorption, m/V = 0.25 g/L, C U(VI) = 60.0 mg/L, I = 0.01 mol/L NaClO4, T = 293 K. (B) The DFT-optimized geometries of the rGOs_uranyl complexes and GOs_uranyl complexes. Reproduced with permission from the publisher.
The microstructures and speciation of radionuclides studied by advanced spectroscopy and theoretical calculation are crucial to understanding the physicochemical properties of radionuclides in the natural environment.
These findings are described in the article entitled Microstructures and speciation of radionuclides in natural environment studied by advanced spectroscopy and theoretical calculation, published in the journal Science China Chemistry. This work was led by Xiangke Wang from North China Electric Power University.








