
First discovered in 1911, the Pictet–Spengler reaction ranks among the most fundamental reactions in organic chemistry. It has found broad applications in the syntheses of indole-derived natural products and pharmaceuticals. For example, a Pictet–Spengler reaction is involved as one key step in the synthesis of Cialis, a drug for treating erectile dysfunction (ED) or benign prostatic hyperplasia (BPH).
However, there has been a long-term debate on the mechanism of Pictet–Spengler reactions. The key issue that remained elusive was the role of spiroindolenine, a widely proposed intermediate of Pictet–Spengler reactions since the early 1950s. How is the involvement of spiroindolenine species related to the reaction outcomes? How does it influence the stereochemistry of the final products? Previous studies did not reach a consensus.
In a new study published in Chem, Chao Zheng, Shu-Li You and co-workers from the Shanghai Institute of Organic Chemistry (SIOC), Chinese Academy of Sciences have provided unified mechanistic understanding on Pictet–Spengler reactions.
The researchers performed theoretical investigations on two prototype models with combined density functional theory calculations and direct molecular dynamics simulations. They disclosed two types of “limiting reaction mechanisms” of Pictet–Spengler reactions.
In one case, spiroindolenine species acts as a “productive” intermediate. That means it can be converted to the final product directly via dynamics effects. The chirality maintenance phenomenon can be expected during this process. In the other case, spiroindolenine species is “non-productive”. Its formation is irrelevant to the overall reaction outcomes. Further experiments supported these theoretical results.
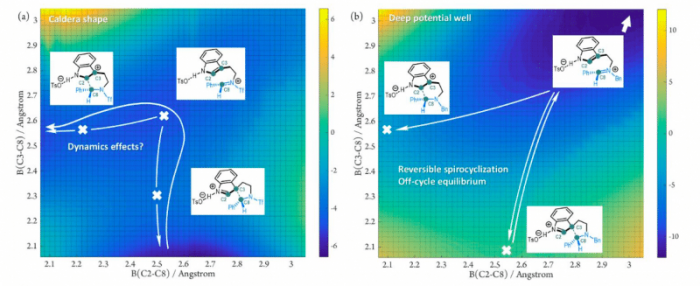
Fig. Potential energy surfaces of prototype Pictet–Spengler reactions: (a) spiroindolenine as “productive” intermediate; (b) spiroindolenine as “non-productive” intermediate.
By applying these results to a series of known catalytic asymmetric Pictet–Spengler reactions, the researchers showed a more comprehensive mechanistic picture. Structural perturbation of substrate or switch of catalyst alters the reaction profile significantly. Overall, the researchers developed a convenient protocol that can make a quick but insightful interpretation of the mechanism of a given Pictet–Spengler reaction.
The upgraded mechanistic insights obtained in this study addressed the long-term issue of the role of spiroindolenine intermediate in Pictet–Spengler-type reactions. This work serves as a vivid example that shows the inherent complexity of organic reactions. The real mechanistic picture of a textbook reaction can be far more complicated than that are usually drawn in schematic line art and can be perturbed by subtle changes in reaction conditions. The future work of this research group will be focused on and further exploring the reactivity of spiroindolenine species and developing novel catalytic asymmetric transformation towards polycyclic indole derivatives which might find potential applications in drug discovery.
These findings are described in the article entitled Unified Mechanistic Understandings of Pictet-Spengler Reactions, recently published in the journal Chem. This work was conducted by Chao Zheng, Zi-Lei Xia, and Shu-Li You from the Chinese Academy of Sciences. This work was financially supported by the Ministry of Science and Technology of China, National Natural Science Foundation of China, Chinese Academy of Sciences, and Shanghai Science and Technology Committee.








