
Carbohydrates can be found in almost all forms of life – they not only serve as an energy source but also covalently attach to other large molecules, such as proteins and lipids. This attachment reaction is called glycosylation, which is a fundamental biological process that can largely increase the diversity of the molecules produced by our cells. Glycosylation is tightly controlled by multiple enzymatic steps within two cellular structures: the endoplasmic reticulum (ER) and the Golgi apparatus.
The surface of our cells is full of the products of glycosylation, including both glycoproteins and glycolipids, that are involved in diverse functions and also play a role in diseases. Some “smart” toxins recognize these cell surface carbohydrates as their entry portals. For instance, Shiga toxins (Stx), produced by Enterohemorrhagic E. coli (EHEC), use a kind of glycolipid called Gb3 (Globotriaosylceramide, also known as CD77), and ricin, produced by the castor oil plant, uses a variety of glycoproteins containing galactose moiety as their receptors, respectively. On the other hand, these toxins can also be used as probes to dig into the molecular process of glycosylation. Now, the question is how to achieve it.
In our recent paper published in the journal PLoS Biology, an advanced technology called CRISPR/Cas9-mediated genetic screen was utilized to study mammalian glycosylation. The screen systematically disrupts genes one by one, and the genes whose disruption renders cells resistant to toxins are identified. As expected, the Stx screen identified almost the entire known pathway of Gb3 biosynthesis; while the ricin screen successfully identified key proteins involved in the N-linked protein glycosylation.
When we compared the results of two screens, one poorly characterized protein that acts as a novel Stx-specific factor, LATMP4A, caught our attention. It is a Golgi protein and specifically required for biogenesis of Gb3, but not for other glycolipids. We further narrowed down its function to a short piece – only containing 32 amino acid residues. Since LAPTM4A colocalizes and physically interacts with A4GALT (also known as Gb3 synthase, the key enzyme for the pathway), we proposed it is potentially acting as a “helper” for that enzyme to make Gb3 (Figure 1).
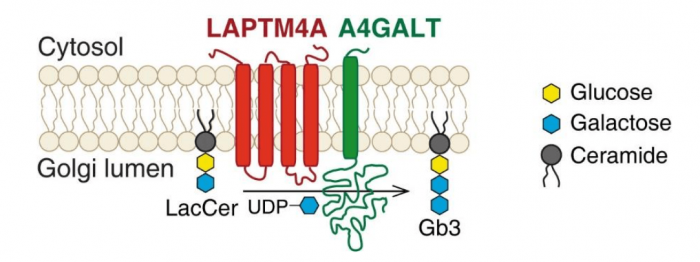
Figure 1. LAPTM4A is required for Gb3 biosynthesis. Lactosylceramide (LacCer) is the precursor of Gb3. A4GALT (α-1,4-galactosyltransferase) produces Gb3 by catalyzing the transfer of galactose from UDP-galactose (uridine diphosphate galactose) to LacCer. This reaction happens in the lumen of the Golgi. LAPTM4A acts as the “co-activator” of A4GALT, which is required for its enzymatic activity. Image republished with permission from PLOS Biology from https://doi.org/10.1371/journal.pbio.2006951
We also found two factors required for both toxins: TMEM165 and TM9SF2. Both are found in the Golgi. When either one was removed from cells, the global level of glycosylation was reduced. They are likely required to maintain a proper Golgi environment for optimal activity of the glycosylation enzymes. We experimentally confirmed that TMEM165 plays an essential role in handling manganese — a metal cofactor critical for many enzymes involved in glycosylation. The role of TM9SF2 remains a mystery. Interestingly, we also found that the cells have difficulties to transport endosomal vesicles when TM9SF2 is missing, although the mechanism remains to be discovered.
In more general terms, our works reveal novel Golgi proteins and provide mechanistic insights to understanding the glycosylation processes. These findings also provide new therapeutic targets for preventing the intoxication of toxins and for understanding glycosylation-related disorders.
This work was described in the article entitled Genome-wide CRISPR screens for Shiga toxins and Ricin reveal Golgi proteins critical for glycosylation, recently published in the journal PLoS Biology.









