
Nowadays, the increasing global demand for clean energy drives numerous research studies on the generation of electric power through efficient and eco-friendly means.
Among these studies, solid oxide fuel cell (SOFC) is a representative clean technology that uses fuel (hydrogen) and oxygen to generate electrical work, and it attracts extensive attention worldwide because of its low greenhouse emissions and high energy conversion efficient (>60%) [1].
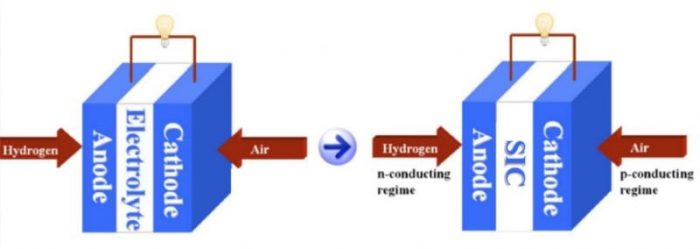
Figure 1. Conventional SOFC (left) based on ion-conducting electrolyte and new SOFC (right) based on a semiconducting-ionic conductor. Credit: Chen Xia
Typically, SOFCs employ an ionic conductor yttria-stabilized zirconia (YSZ) as the electrolytes, which require high operational temperatures (800-1000 °C) to realize a sufficient ionic conductivity for normal fuel cell operation, hindering the widespread application of state-of-the-art SOFCs [2]. Therefore, developing low-temperature (LT) electrolytes is a current mainstream of SOFC research.
Recently, an efficacious strategy based on semiconducting-ionic conductors (SIC) has been developed by incorporating semiconductors into single-phase ionic conductors. These new SIC materials don’t strictly conform to the conventional concept of an electrolyte layer, but exhibit comparable or even higher ionic conduction than the leading level of YSZ.
The corresponding SOFC (Figure 1) takes advantage of the semiconducting properties (e.g., energy bands and p-n junction) to separate charge carriers and the material bi-catalytic capabilities to realize the fuel cell functionality and block the electronic conduction through the membrane layer. These new findings successfully bring the fuel cell operational temperature down to a low-temperature range of 400-600 °C, indicative of a commercial feasibility [3,4,5].
The strategy is new, still waiting and welcoming more materials to such SIC family. Hence, more studies on SIC exploitation and fuel cell device working mechanisms are desired. Motivated by the newly-found ionic conducting behavior in versatile semiconductor ZnO, our recent paper reports a new SIC by introducing ZnO into an ionic conductor, La/Pr-doped CeO2 (LCP), and systematically evaluates the phase structure, morphology, electrical, and electrochemical properties of the materials (LCP-ZnO) and the corresponding fuel cells [6].
Our work first carries out the material characterization study of LCP-ZnO and fuel cells by XRD, SEM, and EDS. The results evidence that LCP and ZnO co-exist in the SIC without any chemical interaction and show the morphology of nano-sized ZnO particles and irregular block-shaped LCP with massive biphasic interfacial regions between the ionic conductor and semiconductor, which is believed to promote the ionic conductivity via interfacial conduction.
Then, in the second part of our work, we investigated the electrochemical performances of LCP-ZnO fuel cells as a function of LCP/ZnO proportion. The fuel cells are assembled into a same symmetrical configuration with Ni0.8Co0.15Al0.05LiO2-δ(NCAL) as both anode and cathode. All LCP-ZnO fuel cells demonstrated good power outputs in the range of 780~1055 mW cm-2along with high OCVs of 1.04~1.07 V at 550 oC. The peak power density is 1055 mW cm-2, exhibited by the fuel cell based on 7LCP-3ZnO. The results manifest the technological feasibility of LCP-ZnO as an electrolyte membrane in LT-SOFC, and the ratios between the semiconductor and ionic conductor constituents strongly affect the cell performance.
Furthermore, the electrical conductivities of three selected composite samples, 6LCP-4ZnO, 7LCP-3ZnO, and 8LCP-2ZnO, were investigated by I-V characteristics and electrochemical impedance spectra (EIS). Our analysis considers that the total conductivity of LCP-ZnO can be obtained according to the simulated ohmic resistance from EIS results, while the ionic resistance is reflected by the slope of polarization curve at the ohmic polarization region. As a result, the 7LCP-3ZnO SIC exhibits a higher ionic conductivity of 0.08-0.29 S cm-1 at 475-550 °C, compared to 6LCP-4ZnO, 8LCP-2ZnO, and pure LCP, indicating that a proper ratio is one of the key factors to reach optimized power outputs.
It is also verified that the 7LCP-3ZnO have mixed ionic conduction and electronic conduction. An additional measurement was conducted to test the proton conducting behavior of 7LCP-3ZnO by using an O2-blocking fuel cell with BZCY blocking layer. The resulting protonic conductivity of 7LCP-3ZnO shows a considerable value of 0.093-0.12 S cm-1at 525-550 oC, suggesting the prepared 7LCP-3ZnO is a hybrid O2-/H+/e–conductor.
It is known from a conventional view that electronic conduction is an unfavorable for SOFC electrolyte, since the short-circuiting issue will cause serious OCV and power losses when electrons transport via the membrane. To interpret the high OCV and output of our LCP-ZnO fuel cell, in the last part, we study the Ni/ZnO Schottky junction of LCP-ZnO half cell at the interface of anodic metal/membrane under fuel cell circumstance. The response currents as a function of bias voltage for NCAL-Ni/7LCP-3ZnO half cell were measured at 550 oC with air and H2flow to the anodic NCAL, respectively. The measured non-reflection response in Figure 2(a)and rectification response in Figure 2(b)certify the in-situ formed Ni/ZnO Schottky junction at the anode side.
Hence, we propose a schematic diagram of the Schottky junctio for describing the electron blocking process in 7LCP-3ZnO fuel cell as illustrated in Figure 2(c). The barrier height of Ni/ZnO junction is able to prevent the electron flow from anode to membrane layer, enabling the high OCVs (≥1 V) and outputs of the LCP-ZnO fuel cell.
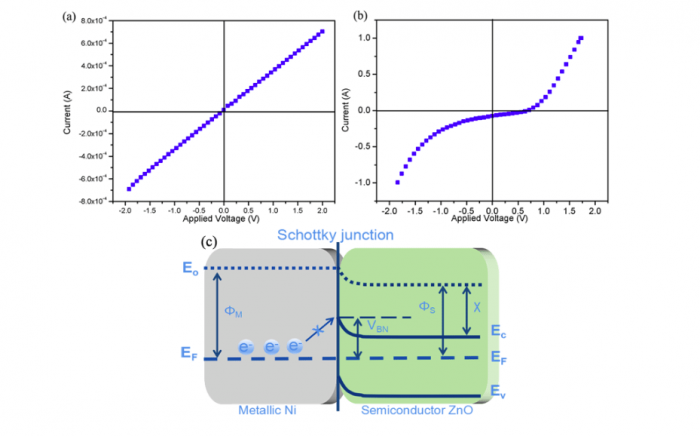
Figure 2. The response current as a function of bias voltage for the NCAL-Ni/7LCP-3ZnO half cell (a) in air and (b) in H2 atmosphere. (c) Schematic diagram of the Ni/ ZnO Schottky junction and energy band structure in LCP-ZnO fuel cell. Republished with permission from Elsevier from: https://doi.org/10.1016/j.jpowsour.2018.04.096
This work is significant because it confirms:
- the validity of semiconducting-ionic conductor as an electrolyte membrane in SOFC;
- ZnO as a multi-function semiconductor is promising for SOFC;
- the hybrid conducting property of the proposed material;
- the in-situ formed Schottky junction in SIC fuel cell.
The above content can be found in the recent article entitled Electrochemical and electrical properties of doped CeO2-ZnO composite for low-temperature solid oxide fuel cell applications, recently published in the Journal of Power Sources. This work was conducted by Chen Xia from the Royal Institute of Technology (KTH) and Bin Zhu from Hubei University and Loughborough University.
References:
- R.M. Ormerod, Chem. Soc. Rev. 32 (2003) 17-28.
- B.C.H. Steele, Solid State Ionics129 (2000) 95-110.
- B. Zhu, R. Raza, G. Abbas, M. Singh, Adv. Funct. Mater. 21 (2011) 2465-2469.
- B. Zhu, B. Wang, Y. Wang, R. Raza, W. Tan, J.S. Kim, P.A. van Aken, P. Lund, Nano Energy 37 (2017) 195-202.
- L. Fan, B. Zhu, P.C. Su, C. He. Nano Energy, 45 (2018) 148-176.
- Z. Qiao, C. Xia, Y. Cai, M. Afzal, H. Wang, J. Qiao, B. Zhu. Journal of Power Sources, 392 (2018) 33-40.








