
How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. This combination creates clusters of soap, water, and grime called micelles.
Soap is a product that most of us use every day, yet most of us also don’t know exactly how it works. Soap just seems to get your hands and the dishes clean somehow. Yet there’s a lot that goes on in how soap makes you clean, and understanding how it works could save you some hassle and some money.
“Soap and water and common sense are the best disinfectants.” — William Osler
How Is Soap Made?
Soap was reportedly first invented around 2800 BCE, by the ancient Babylonians. The first soaps were made out of a conglomeration of water, animal fat and wood ash, and were mainly used to wash wool and cotton. The process has been refined and the ingredients have changed a little over thousands of years, but soap remains largely the same in its formula and application.
Soap is created through the combination of alkali, (a basic ionic salt made out of metallic substances) fats and oils. The alkalis in today’s soaps are usually derived from substances like lye or caustic potash, though they were traditionally made out of ash from wood. The oils found in soap are usually plant-based, like coconut oil, and fats usually come from animal sources like beef tallow.
A process integral to the creation of soap is referred to as saponification, and it involves using the alkali to divide the oils and fats into glycerin and fatty acids respectively. The potassium or sodium found from the alkali is then able to join the fatty acids within these compounds and, as a byproduct, water and soap are produced.
The Function of Soap
Soap’s job is to wash away dirt and grime off of surfaces, whether that be your skin or a dish in the sink. You usually employ soap to wash off substances water itself cannot get rid off. More often than not this is greasy food remnants, which are full of oils. These oils are very difficult to get rid of without soap because water is a polar molecule. This means that water bonds more easily with itself than other types of molecules, like molecules of oil. Oil molecules don’t have poles and they have different electric charges as well, which means that oil will just remain behind as water clumps together and runs off of it.

Grease is difficult to wash off with water alone. Photo: Lucinda44 via Pixabay, CC0
Chemists say that “like dissolves like.” This means that polar molecules can dissolve other polar molecules, but non-polar molecules require other non-polar molecules to dissolve them. Soap is fairly unique in that it can dissolve in both non-polar and polar solvents. Soap contains both polar and non-polar properties, thanks to its formula. The alkaline that went into the soap leaves the soap with a polar head, and the electric charge of this head means that it is “hydrophilic.” A hydrophilic substance loves water and will eagerly bond with it. In this case, the polar head of the soap molecule matches with the hydrogen atoms of water, which have positive charges. Soap’s other component, a fatty-acid tail, is comprised of hydrogen and carbon atoms. Much like the oils which water leaves behind the tail of soap is hydrophobic, and this means soap can adhere to grease and oils.
“What soap is for the body, tears are for the soul.” — Jewish Proverb
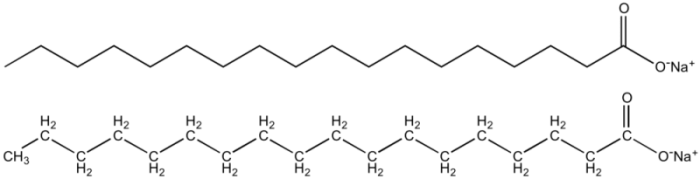
The structure of a soap molecule consists of a polar head with a long fatty acid tail. Photo: Smokefoot via Wikipedia Commons, Public Domain
How Soap Works
These dual-properties allow the molecules in soap to bond with oil and water. Soap can bond to the oil molecules and then pull them away from a surface as it is carried off by water. The soap molecules will pry oil molecules off of surfaces and suspend them within water, which can now wash them away.
Soap also has another function. Soap can decrease the surface tension of water, which allows water molecules to bond easier with a surface, sticking to what needs to be cleaned rather than simply running off of it. Surface tension happens because of the bonds that molecules of water form with other molecules of water. When soap is on a surface along with water the water molecules can spread farther and clean more deeply. These processes are how soap works to make washing away grease and oils that much easier. (Soap can also wash away the oils in your skin, which is why your hands get chapped if you wash them too much.)
Modern soaps may be created with other ingredients in them that serve a variety of functions. Some soaps have certain chemicals added to them which adjusts the pH value of the soap. This can make the soap more acidic or more basic and it can be used to make it safer. Other chemicals can be added to make the soap even more effective at removing dirt and oil. For instance, abrasive compounds can be added to soap which helps it grind down particularly tough grease patches and dirt spots. Enzymes could also be put in soap to help it remove stains from biological sources, like grass stains, by digesting specific fats and proteins.
Difference Between Soap and Detergents
Though many people use the terms interchangeably, there is, in fact, a difference between soap and detergents. Soaps are typically made out of organic, naturally occurring compounds. By contrast, detergents are usually made out of synthetic, man-made materials. Detergents are also usually made out of molecules with a more intense ionic charge, and some detergents can actually dissolve into substances other than water. Detergents and soap are both surfactants, however, which refers to their ability to decrease the surface tension of water.
“Soap and education are not as sudden as a massacre, but they are more deadly in the long run.” — Mark Twain
What About Antibacterial Soap?
Soap is so good at removing substances from your hands that it can also wash away microbes and bacteria. Soap is able to kill and remove bacteria so effectively that there may not be much point to using soap with antibacterial agents included in them. A study done by the University of California found that antibacterial soaps are typically created by just infusing regular soap with compounds like triclocarban or triclosan. These compounds are used in toothpaste and some cosmetics to give them antibacterial properties. The FDA has recently stated that there is little evidence that either triclosan or triclocarban infused soaps are more efficient are removing germs than soap by themselves. In response to the dubious benefit of antibacterial soaps, the FDA has actually instituted a ban on hand soaps that contain antibacterial ingredients, citing that there aren’t enough benefits to merit the inclusion of the substances.








