
It is hard to imagine how electronics would have evolved over the years without the extensive use of semiconductors. These materials, in which the charge of electrons can be controlled, constitute the basis of systems such as transistors and integrated circuits, both of which are essential components in everyday electronic devices. On the other hand, magnetic memory and magnetic data storage, which are key parts of our computers, are also taking advantage of the electronic spins of ferromagnetic materials.
It is therefore natural to wonder if we could make a single material that combines (semi)conducting properties and ferromagnetism while enhancing the current performance of these devices. It is indeed this idea of taking advantage simultaneously of both spin and charge of electrons that led to the development of a new field of research called spintronics. These materials, which combine high-temperature magnetic order and high electrical conductivity, could eventually lead to the miniaturization of the current microelectronic devices down to the nanometer size.
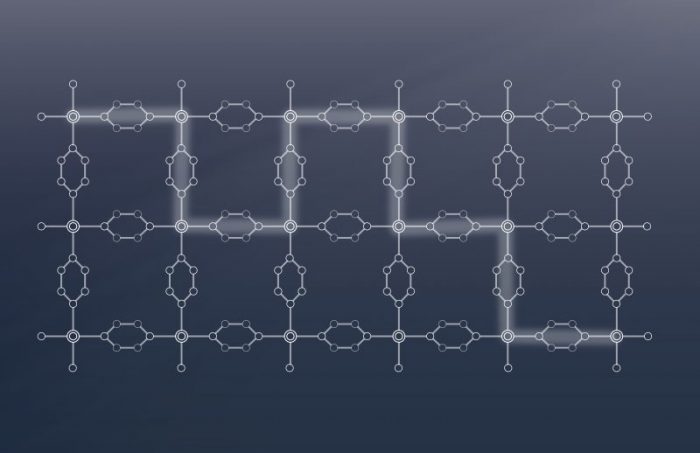
Historically, the systems employed in spintronics applications are usually inorganic solids in which the electronic communication is achieved through direct metal-metal bonding. Despite the success of these materials, the difficulty to chemically tune their properties has led to a shift of interest towards metal-organic materials. Coordination polymers, which belong to this class of materials, are made of metal ions linked by organic bridging ligands, which form extended arrays in one (1D), two (2D), or three (3D) dimensions. The remarkable properties of the well-known graphene and transition metal dichalcogenides have intensified the research interest toward 2D coordination polymers. The ability to optimize their chemical and physical properties through the fine modification of the synthetic conditions, metal ions, organic linkers, and/or framework topology has made them very promising candidates for technological spintronics applications.
Recently, our group demonstrated that unique physical properties appear in the structurally simple 2D coordination polymer CrCl2(pyrazine)2. Although the pyrazine ligand, one of the most commonly-employed ligands in coordination chemistry, does not usually allow for strong magnetic interactions between two metal ion spins, this well-accepted statement is no longer valid when the pyrazine moiety is reduced. The reducing power of the Cr(II) ions results in the transfer of one electron from the Cr(II) centers to the neutral pyrazine ligands, leading to Cr(III) metal nodes and reduced bridging pyrazine ligands in the final CrCl2(pyrazine)2 compound. Experimental and theoretical results show that the electron is smeared over the two pyrazine ligands, giving rise to high electrical conductivity, while the enormous magnetic coupling through the reduced pyrazine scaffold leads to a ferrimagnetic order below 55 K.
Credit: Penny Perlepe
In order to see if these unique properties can also be retained in analogous Cr coordination polymers, we prepared the structurally similar Cr(CH3SO3)2(pyrazine)2 system, where the chlorine ions have been substituted by the methanesulfonate ones. The redox process between the Cr(II) ions and the pyrazine ligands is disabled in this compound, likely due to the change of the redox potential at the Cr site imposed by the presence of the methanesulfonate axial ligands. As a consequence, this material is composed of Cr(II) metal centers and neutral pyrazine ligands.
The magnetic measurements reveal radically different properties compared to the parent CrCl2(pyrazine)2 material. An antiferromagnetic order takes place below 10 K, while the localized electrons, in this case, lead to a gigaohm resistance. This work clearly highlights that the activation of the redox process between Cr ions and the pyrazine ligands in these 2D coordination networks is the key parameter to design of high-temperature magnets possessing high electrical conductivity.
These findings are described in the articles entitled Formation of the layered conductive magnet CrCl2(pyrazine)2 through redox-active coordination chemistry, recently published in Nature Chemistry and Cr(pyrazine)2(OSO2CH3)2: A two-dimensional coordination polymer with an antiferromagnetic ground state, recently published in the journal Polyhedron. This scientific work was conducted by a team led by Rodolphe Clérac at the Centre de Recherche Paul Pascal (Pessac, France), in the frame of an international collaboration including research groups from Denmark, USA, Chile, and France. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 745696.








